Pyrolysis Oil from Biomass: Difference between revisions
mNo edit summary |
m (inserted video) |
||
| Line 1: | Line 1: | ||
{{Category=Biofuel}} | {{Category=Biofuel}} | ||
==Introduction== | ==Introduction== | ||
Pyrolysis Oil is a product of anoxic ("destructive") distillation of biomass. Upon heating of wood or other biomass to sufficient temperature in an air-tight chamber, volatile components are produced. After cooling, some of these are gaseous (hydrogen, carbon monoxide) while others are in liquid form, the so-called '''pyrolysis oil'''. This oil is a dense fuel source - for applications such as heating and steam production. It may be burned by a burner such as the [[Babington_Burner|Babington burner]]. As such, this is a lower-tech substitute for petroleum fuels, with lower caloric value than diesel fuel. At present, it cannot be substituted for diesel in internal-combustion engines due to high viscosity and acidity. Upgrading of bio-oil to Diesel fuel via the [[Biomass_to_FuelFischer-Tropsch|Fischer-Tropsch process]] is possible but may not be practical. Recently, a cheap and open source way to upgrade bio-oil via [[Red Mud and Bio-Oil| Red Mud]] was discovered. | Pyrolysis Oil is a product of anoxic ("destructive") distillation of biomass. Upon heating of wood or other biomass to sufficient temperature in an air-tight chamber, volatile components are produced. After cooling, some of these are gaseous (hydrogen, carbon monoxide) while others are in liquid form, the so-called '''pyrolysis oil'''. This oil is a dense fuel source - for applications such as heating and steam production. It may be burned by a burner such as the [[Babington_Burner|Babington burner]]. As such, this is a lower-tech substitute for petroleum fuels in some applications, with lower caloric value than diesel fuel. At present, it cannot be substituted for diesel in internal-combustion engines due to high viscosity and acidity. Upgrading of bio-oil to Diesel fuel via the [[Biomass_to_FuelFischer-Tropsch|Fischer-Tropsch process]] is possible but may not be practical. Recently, a cheap and open source way to upgrade bio-oil via [[Red Mud and Bio-Oil| Red Mud]] was discovered. | ||
<html> | |||
<iframe title="YouTube video player" width="480" height="390" align=right src="https://www.youtube.com/embed//D8gd2uL1s2U" frameborder="0" allowfullscreen></iframe> | |||
</html> | |||
==Student Projects== | ==Student Projects== | ||
Revision as of 18:08, 6 March 2011
Introduction
Pyrolysis Oil is a product of anoxic ("destructive") distillation of biomass. Upon heating of wood or other biomass to sufficient temperature in an air-tight chamber, volatile components are produced. After cooling, some of these are gaseous (hydrogen, carbon monoxide) while others are in liquid form, the so-called pyrolysis oil. This oil is a dense fuel source - for applications such as heating and steam production. It may be burned by a burner such as the Babington burner. As such, this is a lower-tech substitute for petroleum fuels in some applications, with lower caloric value than diesel fuel. At present, it cannot be substituted for diesel in internal-combustion engines due to high viscosity and acidity. Upgrading of bio-oil to Diesel fuel via the Fischer-Tropsch process is possible but may not be practical. Recently, a cheap and open source way to upgrade bio-oil via Red Mud was discovered.
Student Projects
A great experiment that can be done in a semester is the construction of a simple distillation apparatus to test the procedure with wood chips or newspaper - and to measure the purity and composition of the resulting fuel.
Basic Experiment
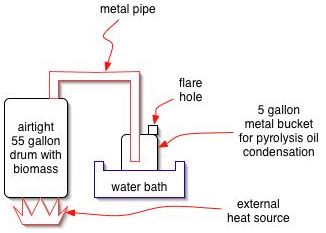
A basic experiment can be performed readily by heating biomass in a 55 gallon metal drum. An external fire may be applied, or an electric heating element may be installed. The vapors that come off can be sent into another drum, submerged in cool water for condensation to occur. A weep hole is put in the second drum for pressure release, and gases may be flared, or captured, at this outlet as the reaction proceeds. When all biomass is distilled, the flare gassing will stop.
The resulting product can then be analyzed.
- Flammability test
- Is fuel readily usable in a [Babington burner]?
- Heating to drive off water
- Heating to drive off lighter fractions to produce fuel oil
- Further heating to provide heavier oils or lubricants
- Cooling to separate phases
- Freezing to separate phases or to separate water
Related Pages
- Paper: "An exploratory study on the removal of acetic and formic acids from bio-oil" Media:BioRes 04 4 1319 Sukhbaatar SIK Expl Removal Acetic Formic Acids Bio Oil 450.pdf
More Information
Contact
contact joseph.dolittle at gmail dot com for more information