Biochemicals from Pyrolysis: Difference between revisions
mNo edit summary |
|||
| Line 53: | Line 53: | ||
3.) So far so good, but then it gets more complicated (depending on bio-oil composition, goals of extraction, etc.). The apolar/oily phase has lighter and heavier components - this is possibly amenable to [http://en.wikipedia.org/wiki/Fractional_distillation fractional distillation]. <br> | 3.) So far so good, but then it gets more complicated (depending on bio-oil composition, goals of extraction, etc.). The apolar/oily phase has lighter and heavier components - this is possibly amenable to [http://en.wikipedia.org/wiki/Fractional_distillation fractional distillation]. <br> | ||
==The alternative: energy== | |||
The alternative to this is always to use these substances for energy. So rather than extract the apolar biochemicals from the oily phase, it may be advisable to mix all of them or certain components with (bio-)diesel (see [[Bio-Oil_/_Diesel_Mixture_Fuels|this page]] for more details about this. Studies have shown that this can be done [http://canmetenergy-canmetenergie.nrcan-rncan.gc.ca/eng/industrial_processes/industrial_energy_systems/publications/200855.html]. Similarly, sugars from the aqueous phase can be fed into an alcoholic fermentation stream if it is not worth extracting them. | |||
==Links== | ==Links== | ||
* excellent, very detailed presentation: [https://noppa.tkk.fi/noppa/kurssi/ke-40.9920/luennot/KE-40_9920_extraction_of_chemicals_from_po.pdf "Separation of chemicals from pyrolysis oil"] by Wytze Meindersma, Eindhoven University of Technology, part of [http://www.biocoup.com/ "Biocoup" - Co-processing of upgraded bio-liquids in standard refinery units] | * excellent, very detailed presentation: [https://noppa.tkk.fi/noppa/kurssi/ke-40.9920/luennot/KE-40_9920_extraction_of_chemicals_from_po.pdf "Separation of chemicals from pyrolysis oil"] by Wytze Meindersma, Eindhoven University of Technology, part of [http://www.biocoup.com/ "Biocoup" - Co-processing of upgraded bio-liquids in standard refinery units] | ||
* Enerkem [http://www.enerkem.com] - biocatalysis | * Enerkem [http://www.enerkem.com] - biocatalysis | ||
Revision as of 20:35, 6 March 2011

Syngas from biomass gasification may contain hundreds of different biochemical substances. Some of these can be quite valuable and therefore simply using the syngas only for energy (example: burning in a Babington Burner) would be wasteful. Depending on the amount of syngas produced, it may be worthwhile to separate out the complex biochemicals from simpler components (e.g. methane, carbon monoxide, hydrogen). The spectrum of biochemicals varies with feedstock and conditions, such as gasifier temperature, duration, pressure etc.
Other components include:
- organic acids (formic acid, acetic acid, propionic acid, butyric acid, etc);
- phenol group;
- carbonyl group (formaldehyde, acetaldehyde, etc.);
- alcohol (ethanol, methanol, etc);
- neutral materials (levoglucosan, acetol, maltol, etc);
- base (substances like ammonia, methylamine, dimethylamine, etc.)
One major component from the dry distillation of wood is acetic acid, which has many applications and can even be used as an organic herbicide. Methanol is another useful and frequent component. Upgrading to biodiesel is possible but may not be practical or even necessary (methanol IC engines are already widely used).
After gasification, the syngas is first cooled down for distillation. As coolants, water or external air may be used. The liquid phase is then diluted with water, which leads to separation into polar/aqueous and apolar/oily layer. Simple, low-tech, open-source methods of separation are needed.
Biological methods for catalysis of CO and H to ethanol are being developed at large scale (e.g. Coskata), which turn the various syngas components into ethanol using a bioreactor. This demonstrates a useful principle: distillation products are further processed using (micro-)biological means.
Possible Applications
- the classic: wood preservative [1]
- organic acids (e.g. formic acid, acetic acid)
- sugars, flavors
- pharmaceuticals
- bioplastics
- fibers, resins, dyes, adhesives
Possible Feedstocks
- various kinds of biomass (corn stalks, straw, wood, leaf litter, algae)
- manure incl. humanure
- animal waste, bones
Important considerations
- The big question is: can this be scaled DOWN to village-scale in a practical way ? If so, the products (incl. biochar from pyrolysis) may become important sources of revenue for the community. Being able to create a large number of different potential products with a single separation mechanism would be significant in terms of autonomy and resilience.
- which feedstocks produce which biochemicals in reasonable quantity ? under what pyrolysis conditions ?
- gasifier design / pyrolysis conditons etc.
- distillation and separation of products; one extraction method is using methanol as a solvent [2]
- further processing of products: when used for energy, purity may be less important then when used for pharmaceuticals (for example).
Relevant Expired Patents
- LEVOGLUCOSAN PRODUCTION BY PYROLYSIS OF PRETREATED STARCHES
- Method and apparatus for converting solid organic material to fuel oil and gas
Possible OSE Algorithm (under development and up for debate)
The goal here is to develop a simple, robust, low-cost, low-energy, (low-tech ?) way to extract certain biochemicals from biocrude. The process really already starts at the time of pyrolysis, since it has major effects on the composition of the resulting bio-oil. Abundant solar heat may be available and could replace other processes (can be used for distillation where applicable).
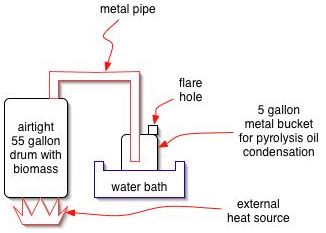
1.) Cool pyrolysis vapors by running them through a coil (copper ?, glass ?, not steel) that is immersed in coolant water. This separates out the gaseous components, namely CO, H2, CO2 and some CH4. Use these gases for energy, perhaps to boil water into steam. Consider a heat recovery system that will ultimately utilize this coolant water in a steam engine (after further heating and boiling)
2.) Dilute the resulting liquid portion with water - this separates the polar aqueous phase from the apolar oily phase; filtration may be required. The aqueous phase has some highly acidic components, such as formic acid and acetic acid.
3.) So far so good, but then it gets more complicated (depending on bio-oil composition, goals of extraction, etc.). The apolar/oily phase has lighter and heavier components - this is possibly amenable to fractional distillation.
The alternative: energy
The alternative to this is always to use these substances for energy. So rather than extract the apolar biochemicals from the oily phase, it may be advisable to mix all of them or certain components with (bio-)diesel (see this page for more details about this. Studies have shown that this can be done [3]. Similarly, sugars from the aqueous phase can be fed into an alcoholic fermentation stream if it is not worth extracting them.
Links
- excellent, very detailed presentation: "Separation of chemicals from pyrolysis oil" by Wytze Meindersma, Eindhoven University of Technology, part of "Biocoup" - Co-processing of upgraded bio-liquids in standard refinery units
- Enerkem [4] - biocatalysis