Pyrolysis Oil from Biomass: Difference between revisions
| Line 10: | Line 10: | ||
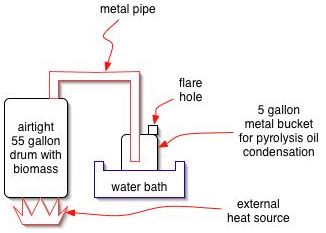
A basic experiment can be performed readily by heating biomass in a 55 gallon metal drum. An external fire may be applied, or an electric heating element may be installed. The vapors that come offcan be sent into another drum, submerged in cool water for condensation to occur. A weep hole is put in the second drum for pressure release, and gases may be flared at this outlet as the reaction proceeds. When all biomass is distilled, the flare gassing will stop. | A basic experiment can be performed readily by heating biomass in a 55 gallon metal drum. An external fire may be applied, or an electric heating element may be installed. The vapors that come offcan be sent into another drum, submerged in cool water for condensation to occur. A weep hole is put in the second drum for pressure release, and gases may be flared at this outlet as the reaction proceeds. When all biomass is distilled, the flare gassing will stop. | ||
[[Image:pyroil.jpg]] | |||
The resulting product can then be analyzed. | The resulting product can then be analyzed. | ||
Revision as of 15:26, 25 September 2008
Introduction
Pyrolysis Oil is a product of anaerobic distillation of biomass. Upon heating of wood or other biomass in an air-tight chamber, the gases that are produced may be liquified by cooling. These gases condense to an oil - pyrolysis oil. This oil may be used as a dense fuel source - for applications such as heating and steam engines. It may be burned by a burner such as the Babington_Burner. As such, this is the lower-tech substitute for diesel fuel - though at present it cannot be substituted for diesel. Both Synthesis and pyrolisis oil are a way towards a biofuel future.
Student Projects
A great experiment that can be done in a semester is the construction of a simple distillation apparatus to test the procedure with wood chips or newspaper - and to measure the purity and composition of the resulting fuel.
Basic Experiment
A basic experiment can be performed readily by heating biomass in a 55 gallon metal drum. An external fire may be applied, or an electric heating element may be installed. The vapors that come offcan be sent into another drum, submerged in cool water for condensation to occur. A weep hole is put in the second drum for pressure release, and gases may be flared at this outlet as the reaction proceeds. When all biomass is distilled, the flare gassing will stop.
The resulting product can then be analyzed.
- Flammability test
- Is fuel readily usable in a Babington burner?
- Heating to drive off water
- Heating to drive off lighter fractions to produce fuel oil
- Further heating to provide heavier oils or lubricants
- Cooling to separate phases
- Freezing to separate phases or to separate water
More Information
Contact
contact joseph.dolittle at gmail dot com for more information