SolarWaterHeater
Objective
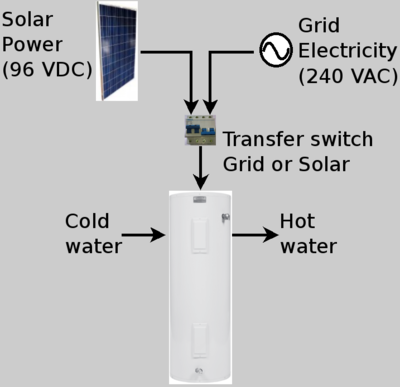
This solar water heater project employs electric commercially available solar panels to collect sun energy and heat water in a conventional electric hot water heater.
The solar panels are conventional solar panels generating DC electricity and the water heater is a commonly available unit normally powered by household AC electricity.
Design
Most electric hot water heaters for household use heat water using a resistive heating element immersed in the water and a simple bi-metallic thermostat. In the United States, they are usually powered by either 120 VAC or 240 VAC. The thermostat usually employs a bimetallic strip with electrical contacts to interrupt the electricity once the desired temperature has been reached and establish electricity flow when the water temperature drops below a threshold.
Tall water heaters often employ two heating elements and thermostats to heat the upper and lower sections of the tank. Shorter water heaters usually have only one heating element.
First, calculate the resistance of the hot water heater heating element, then the solar supply voltage to match this resistance and finally, the number of panels necessary for heating the water.
Test Case
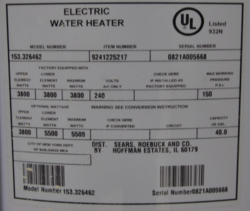
The Unit used for the initial testing is a Kenmore Power Miser 6 with the following specs:
Capacity: 40 gallon Rated voltage: 240 VAC Rated Power: 3800 Watts
The solar panels are Sanyo
24 VDC 230 Watts
The test design is as follows
Water heater resistance:
Power = Voltage * Current (P = E * I) => I = P/V R = E/I = E/(P/E) = 240 Volts / (3800 Watts / 240 Volts) = 15.1 Ohms (Close: It measured 16 Ohms)
Max solar panel power output:
230 Watts 24 Volts 9.5 Amps
Calculate the number of series connected solar panels to match the load at 9.5 Amps:
Supply voltage = R * I = 15.1 * 9.5 = 143.45 Volts #panels = Supply voltage / Panel voltage = 143.45 / 24 = 5.9 Panels
6 panels at max output produce
6 * 230 W = 1.380 kw
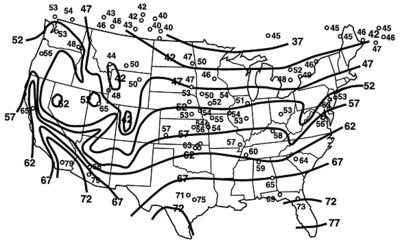
The average water temperature will be the same as the ground temperature, as shown in this illustration:
Calculate the energy required to raise 40 gallons of water from the Dallas ground temperature (19.5 degrees C) to the hot water temperature (~ 51.6 degrees C):
40 Gallons = 151,416 cc energy to raise temperature by 51.6 - 19.5 = 32.4 degrees C:
1 calorie * 151,416 * 32.4 = 4905878 calories or 20539930 watt seconds or 5.705 kwh
Use the power figure above for the 6 panel array to calculate the time required to heat the water:
5.705 kwh / 1.380 kw = 4.134 hours
Summary
It works!