Geopolymers: Difference between revisions
mNo edit summary |
|||
| Line 51: | Line 51: | ||
**Video: [http://www.geopolymer.org/applications/ltgs-brick-low-cost-construction-material Overview: talk by Prof. J. Davidovits] | **Video: [http://www.geopolymer.org/applications/ltgs-brick-low-cost-construction-material Overview: talk by Prof. J. Davidovits] | ||
**Video and technical paper [http://www.geopolymer.org/applications/ltgs-brick-low-cost-construction-material] | **Video and technical paper [http://www.geopolymer.org/applications/ltgs-brick-low-cost-construction-material] | ||
**Dr. Barsoum explains what geopolymer is, how it is made and how it was used in the ancient world [http://www.materials.drexel.edu/pyramids/GeopolymerWhitePaper-Barsoum.pdf} | |||
* http://en.wikipedia.org/wiki/Geopolymers | * http://en.wikipedia.org/wiki/Geopolymers | ||
Revision as of 03:11, 5 December 2009
Introduction
This may be a low-energy, strong brick technology that is also totally decentralizable in production, and may be widely accessible on many continents.
Energy Requirements
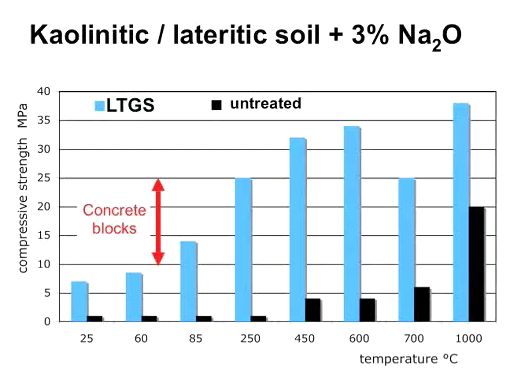
This following graph shows the relationship between firing temperature of geopolymer bricks and compressinve strength. The graph shows that with a firing temperature of 85 degrees Celsius, geopolymers become as s strong as concrete blocks, and with higher firing temperatures, they can be about twice as strong as concrete:
Caveat: there is a requirement of about 2% stabilizer (base salts) for this to happen.
What is it?
Geopolymerization is the process of polymerizing silica and alumina containing minerals with the use of alkali solvents. Discovered (or rediscovered) by Joseph Davidovits, geopolymer cements are likely similar to materials used in antiquity. Although cements are the most common application of geopolymerization, a range of refractory and structural products have been produced. The products of geopolymerization are called poly-silates.
The vast majority of minerals on the earth's surface exist as aluminosilicate crystals (for example: clays, feldspars, quartz). By disolving these and then allowing them to recondense, materials with longer range crystalline structure than its components may be formed.
The most basic inorganic polymerization process uses abundant kaolin clays and silica rich wastes to make a strong cement. A solution of simple aluminosilicates dissolved in an alkali solution acts as a binder, polycondensing upon dehydrolization into a microcrystaline structre which spans the space between silica rich particles. With little technology and at low temperatures, bricks and cements many times stronger than Portland cement are produced. With more intensive technology (artificial atmospheres, high pressures, controlled temperatures) and at hundreds to thousands of degrees celsius, much more structured polymers can be produced which will never combust at any temperature (due to a lack of carbon to react with oxygen), and which also have very low thermal expansion coefficients.
Currently, much of this research is closed source and proprietary. Room temperature setting cements, refractory mineral foams and non-combustible panels for airplanes are amongst the products being researched, and possibly already being widely produced. As the field progresses, more advanced geopolymers and geopolymerizing processes will likely emerge.
The Geopolymer Institute has many useful technical papers on the subject, and has also released several collections of papers from "geopolymere" conferences in France.
Sources of Materials
- kaolinitic clays from subsoil - methods for adequacy analysis needed !
- source of alkali solvents (NaOH) - most common way is through the electrolysis of brine or "salt water," (i.e. sodium chloride in water). Chlorine and hydrogen gases are produced as valuable byproducts. Another source is hard wood ash, which has been used in the past for soapmaking (dissolve ash in rain water, filter out large debris, then boil off the water)
Sample Recipe
The making of alkaline solution, 12 hr before mixing, slowly! disolve 320gm sodium hydroxide (pure lye, as in a drain cleaner) into a liter of water. This should be stirred in slowly, with care, wearing gloves and goggles as it is very caustic. This mix will generate some heat while dissolving.
- After the lye solution is fully dissolved (12hr) mix one part lye solution with 2 1/2 parts sodium silicate. (availible at pottery supplies)
- Basic recipe #8
4 1/2 parts metakaolin 1/2 part lime (type s) 8 parts aggregate (sand mix) alkaline solution as needed (about 1/3 the amount of metakaolin and ash, by weight)
- Mix all the dry ingredients together then stir in just enough alkaline solution to make a stiff mix. Keep the liquid content as low as possible. Cure like concrete, warm and moist.
- Class C Fly ash can replace the metakaolin and lime, if its type F fly ash replace only the metakaolin.
taken from: [1]
External Links
- http://www.geopolymer.org/
- Video: Geopolymer for Newcomers (Prof. J. Davidovits)
- Video: Overview: talk by Prof. J. Davidovits
- Video and technical paper [2]
- Dr. Barsoum explains what geopolymer is, how it is made and how it was used in the ancient world [http://www.materials.drexel.edu/pyramids/GeopolymerWhitePaper-Barsoum.pdf}