Geopolymers
Basics
- This Page goes over Geopolymers, a type of material based off of soil that has been cleared and leveled, then a polymer agent (Typically a liquid) is applied making it into a Composite Material
- Used mainly as either a road surface ( Geopolymer Road ), or as a surface treatment to reduce dust at a facility without using pavement (Typicaly for temporary facilities)
- Can also be used to make Stablalized CEBs
Background
Geopolymerization is the process of polymerizing silica and alumina containing minerals using alkali solvents. Discovered (or rediscovered) by Joseph Davidovits in France, geopolymer cements are likely similar to materials used in antiquity. Although cements are the most common application of geopolymerization, a range of refractory and structural products have been produced. The products of geopolymerization are called poly-silicates.
The vast majority of minerals on the earth's surface exist as alumino-silicate crystals (for example: clays, feldspars, quartz). By dissolving these and then allowing them to recondense, materials with longer range crystalline structure than its components may be formed.
The most basic inorganic polymerization process uses abundant kaolin clays and silica-rich wastes to make a strong cement. A solution of simple aluminosilicates dissolved in an alkali solution acts as a binder, polycondensing upon dehydrolization into a microcrystaline structure which spans the space between silica-rich particles. With little technology and at low temperatures, bricks and cements many times stronger than Portland cement are produced. With more intensive technology (artificial atmospheres, high pressures, controlled temperatures) and at hundreds to thousands of degrees Celsius, much more structured polymers can be produced which will never combust at any temperature (due to a lack of carbon to react with oxygen), and which also have very low thermal expansion coefficients (currently being explored for long-term storage of spent nuclear fuel).
Currently, much of this research is closed source and proprietary. Room temperature setting cements, refractory mineral foams and non-combustible panels for airplanes are amongst the products being researched, and possibly already being widely produced. As the field progresses, more advanced geopolymers and geopolymerizing processes will likely emerge.
The Geopolymer Institute has many useful technical papers on the subject, and has also released several collections of papers from "geopolymere" conferences in France.
Energy Requirements
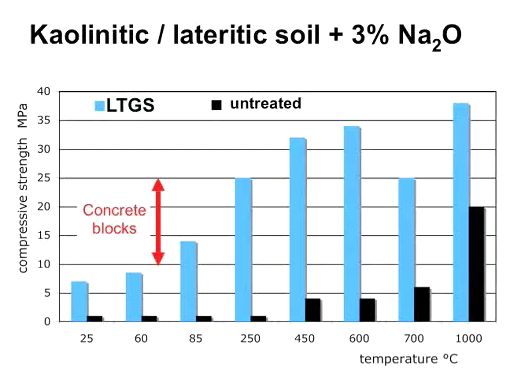
This following graph shows the relationship between firing temperature of geopolymer bricks and compressive strength. The graph shows that with a firing temperature of 85 degrees Celsius, geopolymers become as s strong as concrete blocks, and with higher firing temperatures, they can be about twice as strong as concrete:
Caveat: there is a requirement of about 2% stabilizer (base salts) for this to happen.
Evaluation
This may be a low-energy, strong brick technology that is also totally decentralizable in production, and may be widely accessible on many continents.
Sources of Materials
- a source of silica that can be made soluble in an alkali solvent. This could be a "kaolinitic" clay from subsoil (methods for adequacy analysis needed), diatomaceous earth or volcanic ash. (Note that a handful of kaolinitic clay mixed in a glass of water and allowed to dry completely will leave a discrete hard disc resembling a white poker chip. If it cracks and/or sticks to the sides of the glass is not kaolin.)
- source of alkali solvents (NaOH), - most common way is through the electrolysis of brine or "salt water," (i.e. sodium chloride in water). Chlorine and hydrogen gases are produced as valuable byproducts. Another source is hard wood ash (see page: lye), which has historically been used for soap making (dissolve ash in rain water, filter out large debris, then boil off the water. Or you suspend the hardwood ashes in a dishcloth over a pot and drip the rainwater through.)
- a source of calcium carbonate (limestone, calcareous subsoil, etc.) is generally also used.
Sample Recipe
The making of alkaline solution, 12 hr before mixing, slowly! dissolve 320gm sodium hydroxide (pure lye, as in a "drain cleaner") into a liter of water. This should be stirred in slowly, with care, wearing gloves and goggles as it is very caustic. This mix will generate some heat while dissolving.
- After the lye solution is fully dissolved (12hr) mix one part lye solution with 2 1/2 parts sodium silicate. (available at pottery supplies)
- Basic recipe #8
4 1/2 parts metakaolin 1/2 part lime (type s) 8 parts aggregate (sand mix) alkaline solution as needed (about 1/3 the amount of metakaolin and ash, by weight)
- Mix all the dry ingredients together then stir in just enough alkaline solution to make a stiff mix. Keep the liquid content as low as possible. Cure like concrete, warm and moist.
- Class C Fly ash can replace the metakaolin and lime, if its type F fly ash replace only the metakaolin.
taken from: [1]
Here is a Dr. Michel Barsoum’s formula for a man-made limestone. To a high pH water, add limestone powder, diatomaceous earth and a very small amount of lime. Form and cure at 90 degrees Centigrade. This yielded a stone with a compressive strength > 20MPa and able to withstand 2 months of submersion in water. He stressed that the one of the keys to geopolymerization is the rapid dissolution of silica and that this is best achieved using diatomaceous earth as a silica source, not clay. Because natural earth materials vary, unlike standardized commercial products, the formulae and properties of the final polymerized stone building products may vary greatly from region to region. (Link below.)
Some have described geopolymer as a man-made zeolite; some as man-made limestone. It could also be likened to a man-made soil duripan, especially if volcanic ash is used as a amorphous silica source. Clearly, a range of local natural earth materials can be successfully used, but a period of formula testing is necessary.
See Also
- Ben Magelsen
- Geopolymer Road
- Open Source Soil Stabalization Liquid
- Open Source Soil Stabilizing Vehicle
- Open Source Infastructure Construction Set
Useful Links
- The Wikipedia Page on Geopolymers
- Geopolymer Institute (France)
- Video: Geopolymer for Newcomers (Prof. J. Davidovits)
- Video: Overview talk by Prof. J. Davidovits
- Video and technical paper [2]
- Dr. Barsoum explains what geopolymer is, how it is made and how it was used in the ancient world
- Geopolymer Alliance (Australia)