DME from Methanol: Difference between revisions
Jump to navigation
Jump to search
(Minor Clarification) |
m (Minor Clarification) |
||
| Line 15: | Line 15: | ||
=External Links= | =External Links= | ||
*[http://www.sciencedirect.com/science/article/pii/S0926860X9600275X A 1996 Paper Titled "Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts" ] | *[http://www.sciencedirect.com/science/article/pii/S0926860X9600275X A 1996 Paper Titled "Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts" ] | ||
* | *[http://wiki.gekgasifier.com/w/page/6123698/Dimethyl%20Ether%20%28DME%29 The GEK Wiki Page on Dimethyl Ether (DME)] | ||
[[Category: Biofuel]] [[Category: Bio-Petrochemistry]] [[Category: Energy]] [[Category: Materials]] | [[Category: Biofuel]] [[Category: Bio-Petrochemistry]] [[Category: Energy]] [[Category: Materials]] | ||
Revision as of 20:59, 10 May 2022
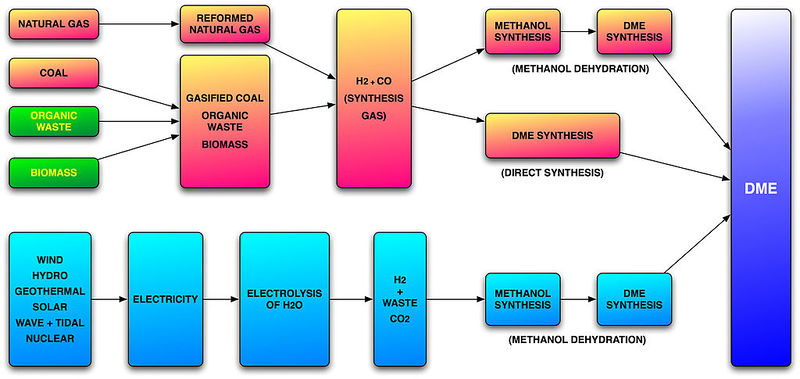
Dimethyl Ether (DME) is created by the dehydration of two methanol molecules methanol across an alumina catalyst.
2 CH3OH --> H2O + CH3OCH3
DME can be created in two steps with the creation of methanol across ZnO and CuO, then its dehydration to DME in the presence of Al2O3.
DME can also be synthesized in a single step which is typically a combination of both catalysts, ZnO/CuO and Al2O3. When the synthesis of methanol and methanol dehydration happen in the same step, an interesting third reaction interaction happens: the water-gas shift.