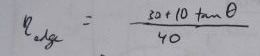Solar Collector Calculations: Difference between revisions
| Line 28: | Line 28: | ||
[[Image:phases of water.gif]] | [[Image:phases of water.gif]] | ||
==Boiling Water (to 100C)== | |||
heat of vap for water is 40.7 KJ/Mol (i assume room temp numbers are good enough here) | heat of vap for water is 40.7 KJ/Mol (i assume room temp numbers are good enough here) | ||
| Line 40: | Line 43: | ||
or thats 1.16 pounds per minute. | or thats 1.16 pounds per minute. | ||
==Generating Steam at 180C/350F== | |||
To raise water from 20C to 180C, which would be a good working pressure of 10 atmospheres or 140 psi [http://www.engineeringtoolbox.com/saturated-steam-properties-d_457.html] | |||
'''Specific heat of water''': 1 Cal/g/C = 1 kCal/kg/C = 4 kJ/kg/C | |||
'''Heat of Vaporization''': 2270 kH/kg | |||
'''Specific heat of steam''': 1/2 Cal/g/C = 2 kJ/kg/C | |||
Total energy re | |||
Revision as of 14:26, 28 June 2008
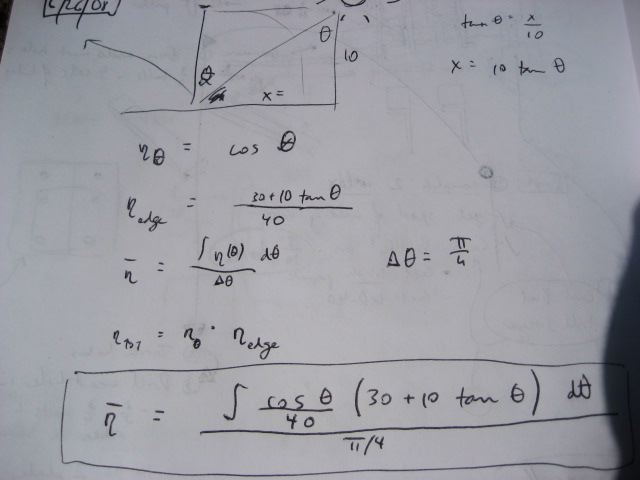
Losses due to edge effects and lack of daily tracking
If we use a passive tracking arrangement for the collector, with only seasonal adjustment, then we have two losses:
- Loss due to the sun not being perpendicular to the array - if theta is the angle measured from the position of the sun at high noon - then the efficiency stemming from this varies as cosine(theta)
- Loss due to edge effects - when the sun is not directly overhead, part of the collector tube is not illuminated (assuming length of collector tube is the same as the length of the reflectors)
The overall average efficiency (eta bar) considering these two losses - for the 6 hours of the day around high noon - is:
This value - I estimate to be around 75% - for the average efficiency of the collector without daily tracking.
Someone please run this on the computer or analytically and fill in the exact value.
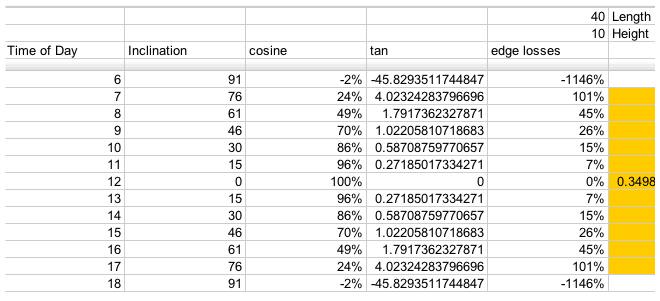
Losses due to edge effects only
For a 40x10 foot solar collector array, with length running East to West, this is a table of losses corresponding to the edge efficiencies (Greek eta_edge) represented by the
term in the above equations:
Vapor Generation Rate
Based on water, we have:
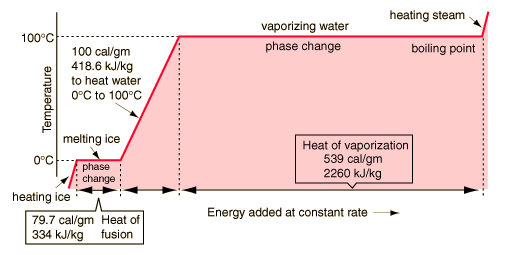
Boiling Water (to 100C)
heat of vap for water is 40.7 KJ/Mol (i assume room temp numbers are good enough here)
1 Kw = 3600 KJ / Hr
1 mol of water is 18 g
20Kw = 72000 KJ/hr * 1/40.7 Mol/KJ * 18 g/mol
gives 31,842 grams of steam produced per hour, or 70 lb per hour
or thats 1.16 pounds per minute.
Generating Steam at 180C/350F
To raise water from 20C to 180C, which would be a good working pressure of 10 atmospheres or 140 psi [1]
Specific heat of water: 1 Cal/g/C = 1 kCal/kg/C = 4 kJ/kg/C Heat of Vaporization: 2270 kH/kg Specific heat of steam: 1/2 Cal/g/C = 2 kJ/kg/C
Total energy re