Polyethylene from Ethanol: Difference between revisions
| Line 6: | Line 6: | ||
==Polyethylene – the current status== | ==Polyethylene – the current status== | ||
Almost all PE today is derived from petroleum. In a very energy-intensive process, a petroleum feedstock is cracked at high temperatures. After distillation and purification in large, capital-intensive facilities, ethylene is produced. This is then polymerized to polyethylene, a process that again involves high temperatures, high pressures and often toxic organic solvents. Clearly not an ideal situation. | Almost all PE today is derived from petroleum. In a very energy-intensive process, a petroleum feedstock is cracked at high temperatures. After distillation and purification in large, capital-intensive facilities, ethylene is produced. This is then polymerized to polyethylene, a process that again involves high temperatures, high pressures and often toxic organic solvents. Clearly not an ideal situation. | ||
==Polyethylene from ethanol== | ==Polyethylene from ethanol== | ||
Revision as of 02:31, 13 July 2011
Main > Materials > Bioplastics
Introduction: Polyethylene
Polyethylene (PE) is a polymer of long chains of the monomer ethylene (IUPAC name "ethene"). It is one of the world’s most common plastics, with a wide range of uses and over 60 million tons produced worldwide every year. Several different categories exist, based on density and branching. Common types are high-density PE (HDPE; plastic # 2) and low-density PE (LDPE; plastic # 4). Polyethylene is not biodegradable, therefore significant environmental issues are associated with its use. Recycling of PE is relatively straightforward. When disposables are involved, every effort should be made to replace PE with biodegradable alternatives. However, resistance to biodegradation can also be a desired effect for some applications. For example, geomembranes are often made of HDPE and are widely used as liners for fish ponds, constructed wetlands and biogas digesters. Excellent chemical resistance of PE allows for widespread use in storage applications. PE is also useful as a material for digital fabrication. It can be used in the RepRap 3D printer.
Polyethylene – the current status
Almost all PE today is derived from petroleum. In a very energy-intensive process, a petroleum feedstock is cracked at high temperatures. After distillation and purification in large, capital-intensive facilities, ethylene is produced. This is then polymerized to polyethylene, a process that again involves high temperatures, high pressures and often toxic organic solvents. Clearly not an ideal situation.
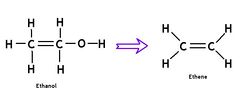
Polyethylene from ethanol
Ethene is a very simple two-carbon organic molecule (C2H4) that does not have to be derived from petroleum. In fact, it can easily be produced from ethanol in a dehydration reaction. This has been known for many decades, but was not cost-competitive at low oil prices. Recently, a Brazilan-Japanese joint venture announced the "Green Polyethylene Project", with sugarcane as the feedstock. Commercial-scale introduction of this "BIO-polyethylene" is planned for 2011. We welcome PE to the club of bioplastics and believe that small-scale production from ethanol can be made practical.
Possible use in carbon sequestration
If renewable energy is used in the polymerization step, bio-PE could even be considered a carbon-negative plastic. Recently, wood-HDPE or bamboo-HDPE composite materials have become popular, combining good structural properties with durability. Taking this idea further, a form of carbon sequestration can be proposed, in which completely dry biomass is stored above ground. Plastic sheets are then used to limit moisture, preventing biodegradation ("plastic-enabled carbon landfill").
Links
- Patent: production of ethylene from ethanol (issued Jan. 1979)
- Patent: process for obtaining ethylene from ethanol (issued Jun. 1987)
- News article: "Brazilian company to make renewable polyethylene"
- Treehugger: "Polyethylene Made From Ethanol 9 Times More Efficient To Source From Sugar Cane, Over Corn"
- Wikipedia entries on polyethylene in general, high-density polyethylene (HDPE) and low-density polyethylene (LDPE)
Collaboration Discussions
Can someone research the patents to the point of proposing a rigorous procedure for producing a test batch of bio-polyethylene, with the hope of scale-up for small-scale production?
Making Ethylene
Dehydration of ethanol seems fairly simple to do with an aluminum oxide catalyst. This method is well suited to small batches and could be easily scaled up to larger batch sizes. It sounds fairly easy to test out. They don't mention the required temperature but it has to be lower than the ignition point of ethanol(~362°C).
If we want food-independent ethylene production, especially for larger scale use, we could go from carbon dioxide and water to syngas (a mixture of carbon monoxide and hydrogen) and then finally to ethylene [1]. This [2] may be useful for producing the syngas.
Polymerization
- Patent: Ethylene Polymerization using a Mixture of Metals and a Halogen as Catalyst (issued Oct. 1961)
- Patent: Ethylene polymer and process for preparing same (issued Dec. 1990)
- Patent: Method for producing an ethylenic polymer composition (issued Jun. 1995)
- Patent: Ethylene polymer and processes for obtaining it (issued 2001)