Polyethylene from Ethanol: Difference between revisions
| Line 22: | Line 22: | ||
==Collaboration Discussions== | ==Collaboration Discussions== | ||
Can someone research the patents to the point of proposing a rigorous procedure for producing a test batch of bio-polyethylene, with the hope of scale-up for small-scale production? | Can someone research the patents to the point of proposing a rigorous procedure for producing a test batch of bio-polyethylene, with the hope of scale-up for small-scale production? | ||
Bio polyethylene background and patent review | |||
Background: | |||
The general route of carbon dioxide to ethylene polymer involves fixation of carbon dioxide into high energy sugar, which is fed to ethanol fermenting yeast and/or bacteria. Ethanol free of water must be obtained and is catalyzed using a fluid bed reactor or recently in a microreactor. The production of a distillation chamber capable of lowering pressure may also benefit the aluminum refining process. Aluminum is a favored catalyst for ethanol dehydration to ethylene but additional compounds such as transition metals increase yield and selectivity while other zeolite catalysts have also been described (Chen et al.). | |||
There are a number of steps involved in polyethylene production from a biotic feedstock; selection of a feedstock, construction of open source vacuum distillers and fluid bed reactors, along with methods of measuring yield and quality of each step will be require bringing a diverse background of knowledge together. | |||
1. Ethanol production | |||
a. Ethanol can be produced on-site or purchased. | |||
b. Sugar cane is the commercially preferred feed stock for the production of ethanol for ethylene production and other industrial uses and has an efficiency 4 times that of corn. | |||
2. Ethanol purification | |||
a. Ethanol can be purified via distillation and has a boiling point of 78.1 C, however the product is azetropic and may not be suitable. Several methods exist and are detailed on wikipedia. Yield of ethanol can be measured via spectroscopic methods with wavelength 2300 cm-1. http://www.erowid.org/archive/rhodium/chemistry/equipment/distillation4dummies.html http://www.umsl.edu/~orglab/documents/distillation/dist.htm | |||
US patent 4,399,000 issued to Tedder on August 16, 1983 details a method to extract alcohols from its water component using an organic solvent to form a lowhead aqueous phase and a high head alcohol-solvent phase. The patent details a device for mixing an extractant with an organic solvent and passage of the solvent-alcohol phase to a vacuum distiller that separates the highly pure alcohol from the solvent. Solvents for use include aryl or alkyl phosphates, a phosphonate, a phosphine oxide, a sulfoxide, a sulfone, an amine oxide or a quarternary ammonium or a phosphonium salt in a ratio of 1 to 10 parts by weight solvent to 1 unit alcohol. | |||
Ethanol to ethylene conversion | |||
Fluid bed reactor allows easy interface solid catalyst and gas phase reagents and separation of gas phase products. Designs can incorporate a number of features for continuous use with features such as catalyst recycling, and constant input and output of substrate and product. Production of ethylene from ethanol and the polymerization of polyethylene from ethylene are carried out in fluid bed reactors and development of this machine would be necessary for both processes involved. | |||
Ethanol’s hydroxyl group can be removed and replaced with a double bond via a dehydration reaction. The endothermic reaction is best catalyzed between 500-700 C and a reactor device capable of controlling the mixing of reaction reagents and catalysts under ideal conditions will be necessary to produce high quality ethylene capable of being polymerized. Steam has been used successfully to provide heat for the reaction and should by modular with the OSE steam generator and solar concentrator. http://www.cheresources.com/invision/topic/7179-ethylene-from-ethanol/ | |||
The reactor device above is detailed in U.S. patent 4,134,926 belonging to Tsao and Zasloff issued Jan 6 1979 which utilizes a fluid bed reactor (11) to contain a catalyzed reaction of ethanol dehydration. The reactor contains powdered catalyst supported on a distributor which can dispense feed ethanol through the catalyst. Ethanol is passed through the distributor in gas phase at 750 C into the reactor chamber at the same temperature. The catalyzed reaction takes place on the surface of the catalyst powder, and the product escapes as gas. The product is equal parts water and ethylene. Catalyst may be removed and loaded into reactor using hopper chambers, which offer the advantage of preparing the new catalyst to ideal temperature and conditions increasing control of the main reactors conditions. According to the patent ethylene yields of 99% are possible with fluidized bed reactors. | |||
Acids or metals may be used for catalysts for this reaction, however aluminum silcate is a catalyst that offers high yields and is easy to obtain and work with. AlO3 is a favored catalyst which will be produced by the soil aluminum extractor. However the addition of other compounds as either supporting material or doping of the catalyst has been found to increase yield and purity. | |||
US patent 4,234,752 issued to Wu et al. on November 18, 1980 details a method of using treated gamma-alumina as a catalyst for the dehydration of alcohols. The catalyst is base treated to remove excess surface acid sites which contribute to isomerization. An inert gas is to transport gaseous alcohol through the catalyst and minimizes side reactions. The described method has been found be effective with primary, secondary, and tertiary alcohols of 2 to 20 carbons and under temperatures 200 to 500 C and pressures of 50 to 3500 kPa. | |||
US patent 4,302,357 issued Nov 24, 1981 to Kojima et al. details a catalyst of high purity aluminum silcate of at least 99.6% purity with a phosphate of group IIa, IIb, IIIa, IVb in the wt% of .05 to 5 with a process for its preparation. Aluminum starting material that is capable of producing aluminum silcate under hydrolysis conditions should be utilized and higher purity organic aluminum salts or metallic aluminum is preferred. The primary factor influencing yield is purity of catalyst but pore volume and specific surface area also affect the reaction and ranges 0.15 to 0.50 cc/g and 100 to 350 m2/g respectively are recommended. Magnesium, calcium or zinc is recommended as a phosphate metal cation. The catalyst should be maintained at a temperature between 300 and 450 C. | |||
A paper published by Chen et al. gives a general description of microreactors, catalyst configurations, and details a AlO3 catalyst with TiO2. Microreactors are small precision engineered devices to mix small reactions with a catalyst and heat. Microreactors may be a more suitable design for OSE specifications over traditional fluid bed reactors and were found to be more efficient than fixed bed reactors in this study. Chen et al found AlO3 doped with 10% wt TiO2 have high ethanol conversion efficiency, ethylene selectivity, and long-term stability of over 400 hrs. TiO2 increases that range of active lewis base configurations in the AlO3 matrix and enhances catalytic activity. Temperatures of 420+ C and ethanol concentration of 30-50% were found to be optimal. | |||
Ethylene yield measurements | |||
Ethylene to polyethylene polymerization | |||
Polymerization from ethylene to polyethylene should be conducted in | |||
Polyethylene measurement | |||
Value adding | |||
Polyethylene recycling | |||
Proposal of action: | |||
1. Production of ethanol on-site from sorghum utilizing yeast fermentation. | |||
2. Construction of distillation equipment capable of operating under vacuum, which could possibly be attached to fermentation chamber. | |||
3. Method for measuring alcohol purity. | |||
4. Dehydration of ethanol using a catalyst and fluid bed reactor. | |||
5. Measurement of ethylene yield and purity using spectroscopic methods. | |||
6. Polymerization of polyethylene from ethylene using transition metal catalyst and fluid bed reactor. | |||
7. Measurement of PE yield and purity. | |||
8. Value adding processes such as tensile polymer incorporation or shaping into useful products. | |||
==Making Ethylene== | ==Making Ethylene== | ||
Revision as of 23:48, 26 February 2012
Main > Materials > Bioplastics
Introduction: Polyethylene
Polyethylene (PE) is a polymer of long chains of the monomer ethylene (IUPAC name "ethene"). It is one of the world’s most common plastics, with a wide range of uses and over 60 million tons produced worldwide every year. Several different categories exist, based on density and branching. Common types are high-density PE (HDPE; plastic # 2) and low-density PE (LDPE; plastic # 4). Polyethylene is not biodegradable, therefore significant environmental issues are associated with its use. Recycling of PE is relatively straightforward. When disposables are involved, every effort should be made to replace PE with biodegradable alternatives. However, resistance to biodegradation can also be a desired effect for some applications. For example, geomembranes are often made of HDPE and are widely used as liners for fish ponds, constructed wetlands and biogas digesters. Its resistance to degradation also warrants its use in the natural gas industry in transporting natural gas underground in high density PE pipes. Excellent chemical resistance of PE allows for widespread use in storage applications. PE is also useful as a material for digital fabrication. It can be used in the RepRap 3D printer.
Polyethylene – the current status
Almost all PE today is derived from petroleum. In a very energy-intensive process, a petroleum feedstock is cracked at high temperatures. After distillation and purification in large, capital-intensive facilities, ethylene is produced. This is then polymerized to polyethylene, a process that again involves high temperatures, high pressures and often toxic organic solvents. Clearly not an ideal situation.
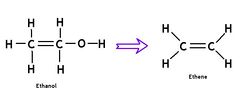
Polyethylene from ethanol
Ethene is a very simple two-carbon organic molecule (C2H4) that does not have to be derived from petroleum. In fact, it can easily be produced from ethanol in a dehydration reaction. This has been known for many decades, but was not cost-competitive at low oil prices. Recently, a Brazilan-Japanese joint venture announced the "Green Polyethylene Project", with sugarcane as the feedstock. Commercial-scale introduction of this "BIO-polyethylene" is planned for 2011. We welcome PE to the club of bioplastics and believe that small-scale production from ethanol can be made practical.
Possible use in carbon sequestration
If renewable energy is used in the polymerization step, bio-PE could even be considered a carbon-negative plastic. Recently, wood-HDPE or bamboo-HDPE composite materials have become popular, combining good structural properties with durability. Taking this idea further, a form of carbon sequestration can be proposed, in which completely dry biomass is stored above ground. Plastic sheets are then used to limit moisture, preventing biodegradation ("plastic-enabled carbon landfill").
Links
- Patent: production of ethylene from ethanol (issued Jan. 1979)
- Patent: process for obtaining ethylene from ethanol (issued Jun. 1987)
- News article: "Brazilian company to make renewable polyethylene"
- Treehugger: "Polyethylene Made From Ethanol 9 Times More Efficient To Source From Sugar Cane, Over Corn"
- Wikipedia entries on polyethylene in general, high-density polyethylene (HDPE) and low-density polyethylene (LDPE)
Collaboration Discussions
Can someone research the patents to the point of proposing a rigorous procedure for producing a test batch of bio-polyethylene, with the hope of scale-up for small-scale production?
Bio polyethylene background and patent review
Background: The general route of carbon dioxide to ethylene polymer involves fixation of carbon dioxide into high energy sugar, which is fed to ethanol fermenting yeast and/or bacteria. Ethanol free of water must be obtained and is catalyzed using a fluid bed reactor or recently in a microreactor. The production of a distillation chamber capable of lowering pressure may also benefit the aluminum refining process. Aluminum is a favored catalyst for ethanol dehydration to ethylene but additional compounds such as transition metals increase yield and selectivity while other zeolite catalysts have also been described (Chen et al.). There are a number of steps involved in polyethylene production from a biotic feedstock; selection of a feedstock, construction of open source vacuum distillers and fluid bed reactors, along with methods of measuring yield and quality of each step will be require bringing a diverse background of knowledge together.
1. Ethanol production a. Ethanol can be produced on-site or purchased. b. Sugar cane is the commercially preferred feed stock for the production of ethanol for ethylene production and other industrial uses and has an efficiency 4 times that of corn.
2. Ethanol purification a. Ethanol can be purified via distillation and has a boiling point of 78.1 C, however the product is azetropic and may not be suitable. Several methods exist and are detailed on wikipedia. Yield of ethanol can be measured via spectroscopic methods with wavelength 2300 cm-1. http://www.erowid.org/archive/rhodium/chemistry/equipment/distillation4dummies.html http://www.umsl.edu/~orglab/documents/distillation/dist.htm
US patent 4,399,000 issued to Tedder on August 16, 1983 details a method to extract alcohols from its water component using an organic solvent to form a lowhead aqueous phase and a high head alcohol-solvent phase. The patent details a device for mixing an extractant with an organic solvent and passage of the solvent-alcohol phase to a vacuum distiller that separates the highly pure alcohol from the solvent. Solvents for use include aryl or alkyl phosphates, a phosphonate, a phosphine oxide, a sulfoxide, a sulfone, an amine oxide or a quarternary ammonium or a phosphonium salt in a ratio of 1 to 10 parts by weight solvent to 1 unit alcohol.
Ethanol to ethylene conversion Fluid bed reactor allows easy interface solid catalyst and gas phase reagents and separation of gas phase products. Designs can incorporate a number of features for continuous use with features such as catalyst recycling, and constant input and output of substrate and product. Production of ethylene from ethanol and the polymerization of polyethylene from ethylene are carried out in fluid bed reactors and development of this machine would be necessary for both processes involved.
Ethanol’s hydroxyl group can be removed and replaced with a double bond via a dehydration reaction. The endothermic reaction is best catalyzed between 500-700 C and a reactor device capable of controlling the mixing of reaction reagents and catalysts under ideal conditions will be necessary to produce high quality ethylene capable of being polymerized. Steam has been used successfully to provide heat for the reaction and should by modular with the OSE steam generator and solar concentrator. http://www.cheresources.com/invision/topic/7179-ethylene-from-ethanol/
The reactor device above is detailed in U.S. patent 4,134,926 belonging to Tsao and Zasloff issued Jan 6 1979 which utilizes a fluid bed reactor (11) to contain a catalyzed reaction of ethanol dehydration. The reactor contains powdered catalyst supported on a distributor which can dispense feed ethanol through the catalyst. Ethanol is passed through the distributor in gas phase at 750 C into the reactor chamber at the same temperature. The catalyzed reaction takes place on the surface of the catalyst powder, and the product escapes as gas. The product is equal parts water and ethylene. Catalyst may be removed and loaded into reactor using hopper chambers, which offer the advantage of preparing the new catalyst to ideal temperature and conditions increasing control of the main reactors conditions. According to the patent ethylene yields of 99% are possible with fluidized bed reactors.
Acids or metals may be used for catalysts for this reaction, however aluminum silcate is a catalyst that offers high yields and is easy to obtain and work with. AlO3 is a favored catalyst which will be produced by the soil aluminum extractor. However the addition of other compounds as either supporting material or doping of the catalyst has been found to increase yield and purity. US patent 4,234,752 issued to Wu et al. on November 18, 1980 details a method of using treated gamma-alumina as a catalyst for the dehydration of alcohols. The catalyst is base treated to remove excess surface acid sites which contribute to isomerization. An inert gas is to transport gaseous alcohol through the catalyst and minimizes side reactions. The described method has been found be effective with primary, secondary, and tertiary alcohols of 2 to 20 carbons and under temperatures 200 to 500 C and pressures of 50 to 3500 kPa. US patent 4,302,357 issued Nov 24, 1981 to Kojima et al. details a catalyst of high purity aluminum silcate of at least 99.6% purity with a phosphate of group IIa, IIb, IIIa, IVb in the wt% of .05 to 5 with a process for its preparation. Aluminum starting material that is capable of producing aluminum silcate under hydrolysis conditions should be utilized and higher purity organic aluminum salts or metallic aluminum is preferred. The primary factor influencing yield is purity of catalyst but pore volume and specific surface area also affect the reaction and ranges 0.15 to 0.50 cc/g and 100 to 350 m2/g respectively are recommended. Magnesium, calcium or zinc is recommended as a phosphate metal cation. The catalyst should be maintained at a temperature between 300 and 450 C. A paper published by Chen et al. gives a general description of microreactors, catalyst configurations, and details a AlO3 catalyst with TiO2. Microreactors are small precision engineered devices to mix small reactions with a catalyst and heat. Microreactors may be a more suitable design for OSE specifications over traditional fluid bed reactors and were found to be more efficient than fixed bed reactors in this study. Chen et al found AlO3 doped with 10% wt TiO2 have high ethanol conversion efficiency, ethylene selectivity, and long-term stability of over 400 hrs. TiO2 increases that range of active lewis base configurations in the AlO3 matrix and enhances catalytic activity. Temperatures of 420+ C and ethanol concentration of 30-50% were found to be optimal.
Ethylene yield measurements
Ethylene to polyethylene polymerization
Polymerization from ethylene to polyethylene should be conducted in
Polyethylene measurement
Value adding
Polyethylene recycling
Proposal of action:
1. Production of ethanol on-site from sorghum utilizing yeast fermentation. 2. Construction of distillation equipment capable of operating under vacuum, which could possibly be attached to fermentation chamber. 3. Method for measuring alcohol purity. 4. Dehydration of ethanol using a catalyst and fluid bed reactor. 5. Measurement of ethylene yield and purity using spectroscopic methods. 6. Polymerization of polyethylene from ethylene using transition metal catalyst and fluid bed reactor. 7. Measurement of PE yield and purity. 8. Value adding processes such as tensile polymer incorporation or shaping into useful products.
Making Ethylene
Dehydration of ethanol seems fairly simple to do with an aluminum oxide catalyst. This method is well suited to small batches and could be easily scaled up to larger batch sizes. It sounds fairly easy to test out. They don't mention the required temperature but it has to be lower than the ignition point of ethanol(~362°C).
If we want food-independent ethylene production, especially for larger scale use, we could go from carbon dioxide and water to syngas (a mixture of carbon monoxide and hydrogen) and then finally to ethylene [1]. This [2] may be useful for producing the syngas.
Polymerization
- Patent: Ethylene Polymerization using a Mixture of Metals and a Halogen as Catalyst (issued Oct. 1961)
- Patent: Ethylene polymer and process for preparing same (issued Dec. 1990)
- Patent: Method for producing an ethylenic polymer composition (issued Jun. 1995)
- Patent: Ethylene polymer and processes for obtaining it (issued 2001)