Polylactic acid/Research Development: Difference between revisions
| Line 34: | Line 34: | ||
purification using electrodialysis] by Madzingaidzo et al examines purification of lactic acid from fermentation broth using mono and bi-polar electrodialysis. An electrical current is applied to the dialysis chamber with mono and bi-layer membrane creating sections holding a base, salt and final acid form. A measurement of % current efficiency (current used to transport molecule from input to concentrated stream/ total current) is used to evaluate the process. | purification using electrodialysis] by Madzingaidzo et al examines purification of lactic acid from fermentation broth using mono and bi-polar electrodialysis. An electrical current is applied to the dialysis chamber with mono and bi-layer membrane creating sections holding a base, salt and final acid form. A measurement of % current efficiency (current used to transport molecule from input to concentrated stream/ total current) is used to evaluate the process. | ||
[http://www.google.com/patents/US2350370 | [http://www.google.com/patents/US2350370 Lactic acid purification] issued to Schopmeyer June 6 1944 covers a method to purify lactic acid from fermentation broth by the use of calcium carbonate salts and esterification with methanol for fractional distillation. The fractional distillation set-up includes a boiler containing methanol and lactic acid and a catalyst (H2SO4) that delivers vapors to a fractionation column that allows the separation of a concentrated lactic acid liquor of ~10-50%. The salting out of calcium lactate uses calcium sulfate which is concentrated and converted to acid form through treatment with sulfuric acid, calcium sulfate forms an insoluble fraction. The concentration was usually 40-60% lactic acid and ethanol can also be used as the esterification. The esterification procedure uses a steam jacket with the following substrate mole ratio 1.5:1:0.005 methyl alcohol: lactic acid: sulfuric acid. Start-up of purification uses 80% lactic acid substrate (depending on concentration), 20 methanol, and a small amount of catalyst. Steady-state is maintained by addition of substrates and catalyst, and recycling of methanol. [[File:US2350370.png | US patent 2,350,370 | Right | 200 pixels]] | ||
Revision as of 13:24, 22 May 2012
Literature review
Process Review
http://naldc.nal.usda.gov/download/4048/PDF L (+) lactic acid fermentation and its product polymerization by Narayanan et al reviews the production of lactic acid and its use as a plastic monomer. The synthetic route of lactic acid is four steps that involve fixating an activator cyanide group to an acetylaldehyde to form lactonitrile, hydrolysis of lactonitrile with sulfuric acid to yield lactic acid and ammonium salt. For purification via reactive distillation lactic acid is esterified with methanol to methyl lactate and water, methyl lactate is distilled, and hydrolyzed to lactic acid with the addition of water. The production of lactic acid from biological sources is through the fermentation of high energy carbohydrates to lactic acid by Lactic Acid Bacteria. Lactic acid is neutralized and precipitated with calcium hydroxide. Calcium lactate is collected and hydrolyzed with water. For purification lactic acid is esterified with methanol to methyl lactate and removed via distillation, before hydrolysis with water. Has use as hardener for cellophane.
"The choice of an organism primarily depends on the carbohydrate to be fermented. Lactobacillus delbreuckii subspecies delbreuckii are able to ferment sucrose. Lactobacillus delbreuckii subspecies bulgaricus is able to use lactose. Lactobacillus helveticus is able to use both lactose and galactose. Lactobacillus amylophylus and Lactobacillus amylovirus are able to ferment starch. Lactobacillus lactis can ferment glucose, sucrose and galactose. Lactobacillus pentosus have been used to ferment sulfite waste liquor." Lactobacillus also have complex nutrition requirements. Rhizopus oryzae are also stereoselective LAB as well as yeasts such as Saccharomyces cerevisiae and Kluyveromyces lactis and have been investigated for their usefulness. Lactase enzymes are stereospecfic and heterolactic species have two isoforms, some species induce their second enzyme only under high concentrations of lactic acid. Genetic engineering on lactobacilli has shown success in controlling stereospecficity of products, reaction rate and yield; Rhizopus oryzae is also under study. Favorable feedstocks are high sugar or starch plants. Techniques to increase yield include pretreatments, simultaneous saccharification, and nutrient supplementation (especially nitrogen - yeast extract). Methods to remove lactic acid product from the fermentation batch include ion-exchange resins and electrodialysis.
Different bioreactor configurations have been studied and batch-wise and continuous reactor sketches are provide. Continuous cell recycle reactors have shown high performance and utilize membranes to retain cells while removing media. Cell immobilization by biofilm establishment shows higher performance to free floating culture systems. High cell concentrations make it much more difficult to maintain optimal conditions in all parts of the reactor and can stress the cells (stereoisomerization). "(a) lowering down of the pH of fermented broth (3.0 to 4.2); (b) Use of hydrophilic membrane and the volatile amine weak base (VAWB) to separate lactic acid from the fermented broth through the hydrophillic membrane to VAWB; (c) Regeneration of lactic acid from salts of weak amine base by selectively vaporizing the volatile amine base. This process can be repeated to ensure the efficient separation of free lactic acid and its salt. "
Polylactic acid technolgy by Henton (2005) reviews production, purification, and polymerization. There is information on Cargill Dow's plant capacities who dominate the market (economic significance), largest produces 400,000,000 lb and produces over half the market. Clostridium thermoaceticum is the highest yielding fermenter but requires pH control. Purification technologies utilize a variety of characteristics of lactic acid to separate it from the broth. Cell and macromolecule filtration followed by electrodialysis that is fed to distillation column, reactive distillation). Tin octoate is the basis catalyst for lactide polymerization which converts LA to stereospecific form. PLA characteristics include crystallinity which affects Tg and Tm.
Lactic acid bacteria
Fermentation procedures
http://144.206.159.178/FT/158/86077/1455313.pdf http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1058040/pdf/applmicro00350-0046.pdf
Lactic acid purification
| Optimization of Batch Reactive Distillation Process: Production of Lactic Acid by Edreder (2010) develops a model for esterifying lactic acid with methanol and distills the methyl lactate, the lactic acid is recovered by hydrolysis. The purity analyzed was 80-99% molefraction, it took 4 refluxes to theretically reach the highest purity.
[http://144.206.159.178/FT/549/63720/1083859.pdf | Process development and optimisation of lactic acid purification using electrodialysis] by Madzingaidzo et al examines purification of lactic acid from fermentation broth using mono and bi-polar electrodialysis. An electrical current is applied to the dialysis chamber with mono and bi-layer membrane creating sections holding a base, salt and final acid form. A measurement of % current efficiency (current used to transport molecule from input to concentrated stream/ total current) is used to evaluate the process.
Lactic acid purification issued to Schopmeyer June 6 1944 covers a method to purify lactic acid from fermentation broth by the use of calcium carbonate salts and esterification with methanol for fractional distillation. The fractional distillation set-up includes a boiler containing methanol and lactic acid and a catalyst (H2SO4) that delivers vapors to a fractionation column that allows the separation of a concentrated lactic acid liquor of ~10-50%. The salting out of calcium lactate uses calcium sulfate which is concentrated and converted to acid form through treatment with sulfuric acid, calcium sulfate forms an insoluble fraction. The concentration was usually 40-60% lactic acid and ethanol can also be used as the esterification. The esterification procedure uses a steam jacket with the following substrate mole ratio 1.5:1:0.005 methyl alcohol: lactic acid: sulfuric acid. Start-up of purification uses 80% lactic acid substrate (depending on concentration), 20 methanol, and a small amount of catalyst. Steady-state is maintained by addition of substrates and catalyst, and recycling of methanol. 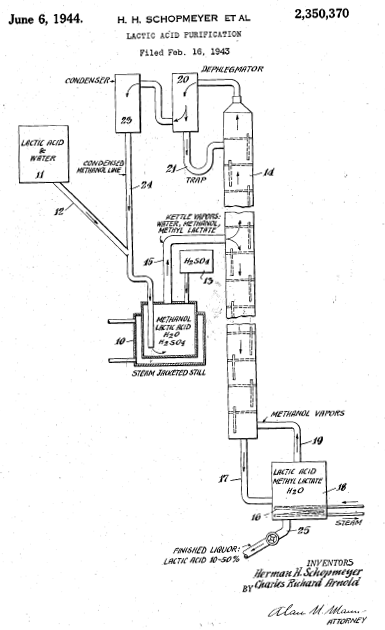
http://www.google.com/patents/US4771001
Polylactic acid polymerization
Synthesis of polylactic acid by direct polycondensation under vacuum without catalysts, solvents and initiators by Achmad et al details a procedure. The authors recommend PLA be pursued using processing plants capable of fermenting feedstock, purifying lactic acid, and condensing the product as is proposed for the OSE product ecology. Streptococcus bovis is a LAB suggested for use but the species is also linked to pathogenicity. The process used by the researchers used three phases for treating the lactic acid and polymerizing its monomer: distillation, oligomerization, and polymerization. The reaction was carried out in 4 l sealable flasks, on magnetic stirrers and heaters, with temperature and pressure probes, and connected to a pressure regulator. During distillation sample temperature is brought to 150 C over 90 minutes and maintained for 60 minutes and PLA concentration increases from 90% to 100% as measured by acid-base titrations. Oligomerization phase was a reduction in pressure to 10 mmHg and temperature was raised to 200 C. The polymerization step was maintenance of the reduced pressure and temperature for 89 hours. The condensate was also separated with gel filtration chromatography and measured with a RI detector. Fourier Transform Infrared Spectroscopy was used to analyze molecules functional groups.
[http://144.206.159.178/FT/862/34857/596552.pdf | Melt/solid polycondensation of l-lactic acid: an alternative route to poly(l-lactic acid) with high molecular weight] by Moon et al (2000) describes a method that yields high weight PLA on the order of 500,000 daltons through a condensation reaction using a tin chloride dihydrate/p-toluenesulfonic acid binary system. They report that reaction temperatures below the Tm (melting point) of PLA yields a better product and is referred to as melt/solid polycondensation. oligo(l-lactic acid) (OLLA) is mixed with tin(II) chloride dihydrate (SnCl2) (0.4 wt% relative to OLLA) and p-toluenesulfonic acid (TSA) (an equimolar ratio to SnCl2). The mixture is heated to 180 C and the pressure reduced to 10 torres over the period of an hour followed by maintenance for 5 hrs. The product consisting of 20,000 dalton polymers is ground and heated to 105 C under vacuum for 1-2 hr to crystallize the polymers. Solid-state post-polycondensation was initiated by increasing the temperature to 150 C and reducing the pressure to 0.5 Torr. Treatment was continued over 30 hours but molecular weight peaked between 2-10 hours and drastically reduced after 20 hours. The results showed a method to obtain high molecular weight PLLA with comparable characteristics to the lactide ROP synthesis. The method used here catalyzes the first step of dehydration to form lactide and the lactide ROP step follows. The high activity of the catalyst and the ability to move through the amorphous PLLA may be driving the reaction by concentrating ester tails and catalyst in the amorphous regions during crystallization.
Polylactic acid value adding
(Poly)lactic acid: plasticization and properties of biodegrable multiphase systems by Averous (2001) experimented with measuring the properties of PLA prepared with different plasticizers. Plasticizers included: glycerol, polyethylene glycol, citrate ester, PEG monolaurate, and oligomeric lactic acid. various mixtures of PLA with thermoactive starch polymers (TSP) were prepared and tested. Plasticizer treated samples show a decrease in Tg (glass transition temp) and therefore Tm (melting temp). Oligomeric lactic acid followed by low molecular weight polyethylene glycol were effective plasticizers while glycerol was ineffective. Finding effective methods to combine PLA and TSP would enhance the product.
http://www.e-polymers.org/journal/PAT2005ePolymers/page/Oral%20Presentations/Section%20B/Martino_Ver_nica_Patricia.pro.1728860278.pdf looks at the use of 4 plasticizers to increase beneficial characteristics for film.