Talk:Nickel-Iron Battery
On toxicity and other safety concerns:
Nickel oxide/hyroxide is indeed toxic, but as it would be contained in a battery, it seems this is likely the best place for such a substance to be. Basic safety protocol should keep anyone who should have to handle the battery and/or its components safe from harm.
Lye isn't 'toxic' it's caustic and corrosive. Either way, it doesn't get along with the body very well. However, once built, the lye will be diluted in a solution (ideally of glycerol), and so there shouldn't be much danger at all associated with the battery once built. However, utmost caution should be exercised when mixing the electrolyte (which shouldn't be a problem if waste glycerine from biodiesel production is used).
Unfortunately, nickel is also a strategic metal, which does add to its cost, but further research may point the way to better localized sources. Of course, this shouldn't be an issue as each 1kWh battery has a one time cost of 2kg nickel (as is explained in the page). All in all, I think NiFe chemistry is about the safest practical battery that could be constructed. Colin 14:45, 16 May 2011 (PDT)
Feel free to edit the section I added on toxicity, I just wanted to make sure that something was there. Yes, lye is corrosive, but I do agree that handled correctly, it can be safe. We might want to add links/comments to the effect that lye can be made from wood ashes.
You might want to do a bit of sourcing for nickel. I wonder how much it costs. - Mark J Norton
I was curious, so I did a quick search and ran the numbers myself. $251.50 for 2kg of nickel. - 5/16/11 at 9:05pm PDT
On sources of nickel: because of cost, I have been thinking about the best starting material for nickel. It seems best to me to start with nickel hydroxide mixed in with the electrolyte, in the appropriate concentration. A small amount of something like nickel nitrate might be needed to provide a thin coat of nickel metal, but I think that the hydroxide by itself should work. In either case, it makes the most sense to start with nickel, and treat it with the appropriate acids and bases to get the form we want. This way, we could even start with nickel shavings, ingots, blanks, or any other form of nickel. Colin 20:47, 24 May 2011 (PDT)
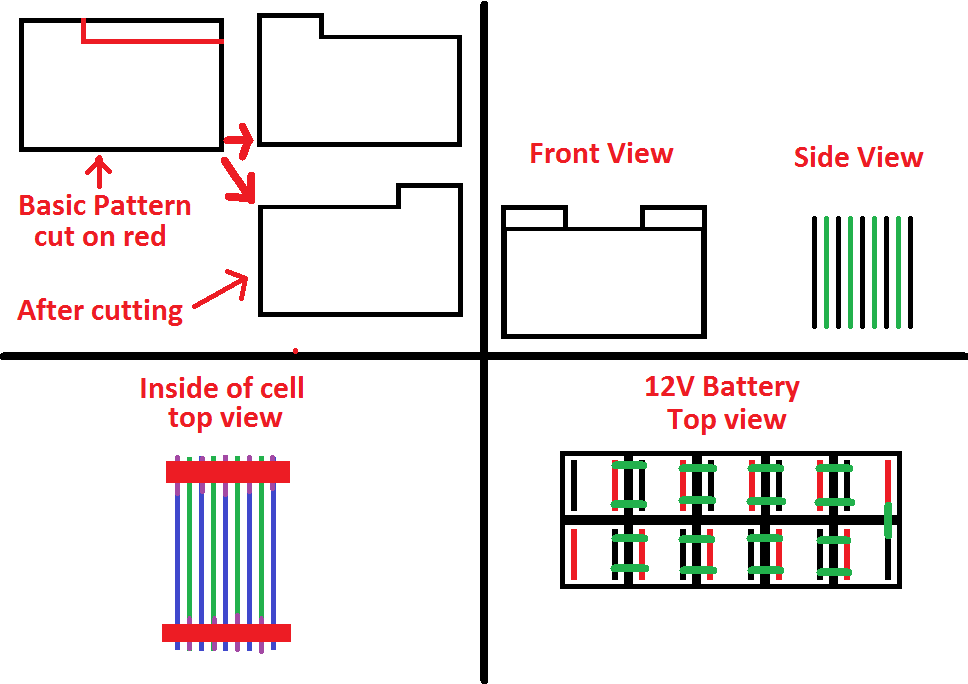
Let me elaborate on the above. The construction technique I'm envisioning for this battery involves a number of plates, all made from relatively thin sheet steel. Half of which would be plated by nickel oxyhydroxide in the first charging cycle, forming the cathode of each 1.2V cell. The steel should be carbon steel with minimal to no content of other metal. I intend on writing this into the article, but I think that it needs graphics to go along. So, the idea is that the sheets which will be the electrodes would have a tab cut out of a corner with the cnc torch table. Then, these sheets will be placed with the remaining tab (like a tab on a folder) of each plate alternating in a cell. This leaves two sets of 'tabs,' one set will be for the anode, and the other for the cathode. With this design, two long steel bars can be welded to the exposed tabs, which should allow for high current capacity, resilience, and rigidity in design. These two bars would be inside the profile of the box when viewing it from the top, so many cells can be placed snugly adjacent to each other, and then welded in series to form a 12V stack. Such a setup should easily handle the highest loads this battery should be capable of driving. Colin 20:55, 24 May 2011 (PDT)
I've added an mspaint rendition of what such a battery would look like in concept. If anyone possessing proper CAD skills wants to improve it, please go ahead and replace it. Colin 22:18, 24 May 2011 (PDT)
The added information to the article is greatly appreciated, but tends to be overwhelming. At this point, I suggest this article is broken into two segments: chemistry and construction details. Anyone else agree with me? Colin 21:26, 24 May 2011 (PDT)
The thing is that this is the development page not an encyclopedia entry, though. I don't think we can afford to oversimplify if we are going to produce something which can compete well with commercial models. The construction and chemistry are intimately intertwined. gregorgregortheinventor
The chemistry and details of construction are not oversimplified in this article. I am not suggesting removing the additional information--I am suggesting creating a new article to house it. Leaving it here with construction information leaves the article extremely cluttered. Colind 19:13, 20 June 2011 (PDT)
I was looking at the diagram showing the battery construction and see that in the top view the plates forming the cells are arranged in 2 rows of "boxes" with some number of boxes along the battery depending on how big it is. Apparently the batteries can produce a fair bit of heat when they are operating and it would therefore be nice to be able to cool them. If you made each of the boxes watertight along the long axis, but allowed it to flow from one box to the next along the rows you could push electrolyte from left to right on that picture. An opening on boxes on the ends which connects across the middle wall would allow fluid to flow either clockwise or counter clockwise slowly around the battery. Given the way the electrodes are arranged flow clockwise would circulate the electrolyte from anode to cathode and the other way would go from cathode to anode. Depending on which way you circulated the fluid the battery would either be easier to charge or easier to discharge. The whole cycle can be driven by slow speed fans or pumps in the battery, making the fans reversible would let you always pump the cooling fluid in the direction that favors what you are doing over the long term (either charging or discharging). As the fluid circulates around the loop you divert some of it through a heat exchanger to cool the battery, the flow should keep most of it at roughly the same temperature even at slow flows I think (you would want the flow slow anyway as I think very fast at all and the ion transport rate would be way faster than the reaction rate and it would mix too much. Anyway, just a thought I had while looking at the diagram. -Andrew Buck 20:34, 2 June 2011 (PDT)
I don't think overheating will be a problem from what I have read. A lot of the energy wasted goes into producing h2 and o2. The catalytic converter will get hot though, especially due to it's small size and during overcharge, and this is something that will need to be addressed.
Of course a decent CAD package should be able to do the thermal modeling, so when we have the homework done and can move on to the design stage that is one of the things that should be given due consideration. But from basic principles I wouldn't expect it to be a problem, a vertical surface looses about 25 watts per degree to convection in air at around room temp so say it was a 1Kw battery which has a surface area of a meter, and the charge efficiency is 80% (which is what the changhong batteries are) then we charge it at 0.2 C rate well-past worst case scenario in a photovoltaic system (since the batteries are usually much larger than the solar collector peak output) that's 200 watts *0.8 =16 watts. the surface are of a 1Kw hour battery, the energy density is around 30 Wh per liter according to wikipedia (which is just a ballpark since it will vary substantially with design of course) so 33 liters for a 1 kWh battery if i is a cube then 32 cm on a side , suppose the 4 sides and total are 5000 sq cm that is 0.5 square meters.
If it is say polyethylene which is about 0.4 W/m*K [1] and say worst case 5 mm thick. Then that is 0.5/0.005*0.4=40 W/K so it would not be a problem. Temperature rise might be less than a degree above ambient. -gregor
The cells are isolated to prevent shorting between cells. Were the electrolyte allowed to flow freely between cells, the end result would not be a series stack, so a highly inefficient 1.2V battery would result. Colind 19:13, 20 June 2011 (PDT)
One idea might be to use a colloidal form of CaOH in a noble gas atmosphere with suspended Anatase titanate nanotube (very easy to produce) homogeneous throughout the gel, but that would have to be tested and evaluated.--James Clark 06:47, 11 June 2011 (PDT)
So many suggestions here seem to fall within the realm of 'high tech.' Remember that this battery was very successfully built and marketed in the early 20th century. For the purposes of OSE, there is no need to complicate. Simpler construction also means more reliable and resilient use. Complex electrode geometries tend to mean degradation over time. A classic example are the 'fingers' or 'tendrils' formed in lead acid batteries which will ultimately short them out. Of course, additives can mitigate the problem, but it would be far simpler just to avoid it all together and aim for a reasonably high surface area, but a high capacity battery. This would result in a more robust solution which would easily store 1kWh or possibly even much more. The size and weight of the battery are not critical factors. Marcin informed me that their primary use will be in storing solar/wind energy for off-grid applications. So much of the information added to this article is irrelevant to that purpose. Colind 19:13, 20 June 2011 (PDT)
Sustainability One thing that haven't been addressed as far as I can tell is recycling which will also help bringing cost down over time. In order for any technology to be sustainable you need a cradle to cradle approach. So my question is: When NiFe cells gets worn out how do we reclaim materials especially the nickel? --Bhm 03:18, 9 June 2011 (PDT)
The great thing about NiFe chemistry is that it does not tend to degrade very much. NiFe batteries built 50-100 years ago are still in operation today, working just as well as they did then. Even in the event of a rebuild, the chemistry should be simple enough to recover the vast majority of the Nickel in the battery. Colind 19:13, 20 June 2011 (PDT)
sustainability: There should be no problem with that no matter how we work it as long as no toxic chemicals are used. Just grind the cells up or dismantle them and take the material out and replace with new stuff. Might have to replace the pockets too if the nickel comes off.
Okay, actually while I'm here, I have been meaning to give a status report because it looks like I am more or less done with this section of the project as my time is now being consumed trying to get some progress on some of the organizational issues.
I moved it to the "current progress section" Gregor 23:21, 22 June 2011 (PDT)
Proposed prototype
A practical plan for the prototypes and eventual production (the process for which is more intimately intertwined with the design than it is for e.g. the lifetrac) will be produced after the documents listed in the sticking points section are obtained and read.
The reason these relatively difficult to make electrode geometries are used instead of just plates is (mostly) in pursuit of higher active material utilization (major impact on cost) and higher discharge rates, and reasonable energy and power density.
It is possible to make a battery from active nickel oxyhydroxide particles of 200 mesh that is rated at 0.1 C or so, which is about 0.075 mm. The surface are is one of the main determining factors for the reaction rate, and therefore the acceptable discharge rate (although I guess a plate might decrease the resistance between the current collector and the active material which is another significant factor).
But if you have a surface(backed by metal) then the ratio of volume/area is T^3/T^2=T, where T is the thickness, whereas with a sphere it is (pi*d^3/6)/(pi*d^2)=d/6 where d is the diameter, thickness of the layer would have to be therefore 1/6th of 0.075 mm to have the same volume/surface area with a plate as a particle, or 0.0125 mm, 12.5 microns. If you use both sides of the plate then you get 0.0025cm*1cm^2=1/4000 cm^3, density of nickel hydroxide is 4 g per cc, so the weight of material per sq cm of plate is 1 milligram. You would get 10 grams per square meter of plate, which could be increased slightly (depending on plate thickness) with perforations, and you could make the plate foil 0.01 mm thick, but you still would need a lot of foil. It would have to be packed together in some way to achieve reasonable density, and plating it with nickel would be that much more work.
Also that 200 mesh is with cobalt compound additive to improve conductivity of the active material mass and the contact resistance between the particles, which could change things a lot. Without it the layer would have to be thinner to get the same internal resistance. How much needs to be verified by finding the conductivity of the material with and without additive. Secondly those documents listed below will provide information about exactly what sort of surface area to volume ratio leads to what sort of battery characteristics, so we can decide if a higher v/a ratio may be acceptable.
Also, there is no need for a self protection mechanism, and you can't have high current density and and current limiting at the same time. Lower internal resistance is always good, within the range that is attainable.
Lastly the nickel hydroxide would probably fall off the electrode, unless held in place in some way. With a foam or other porous mesh-like electrode that is overcome with the mechanical support that the matrix provides.
I took this off the main page and moved it here because it is more chatty really though maybe some parts shoudl be integrated witht eh future prototypes section. Gregor 21:08, 24 June 2011 (PDT)