User:Dorkmo/Ideas/Battery
< User:Dorkmo | Ideas
Jump to navigation
Jump to search
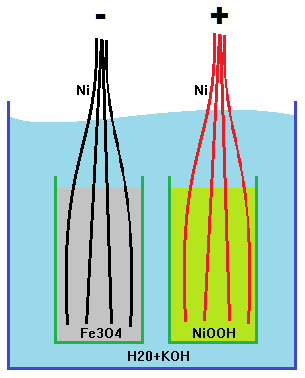
idea is to use powder contained in tubs. the walls will have a perforated mesh whos holes angle up and out so that the powder cannot escape. Nickel wires will be placed in the powder to conduct out of the cell. -Contact area is not optimal - that's why they use liquid electrolyte?-MJ
design
principles
- maximize 3d printing
- utilize parametric customization
- avoid complicated chemical processing
- no nickel plating
ideas
- powders are very likely to become airborne
- internet has suggested that "black strap molases" was added to edison batteries
- might try to mix in at different ratios to see what happens to consitency
- add a couple super capacitors to get some cold cranking amps?
- not sure if that would work but just an idea
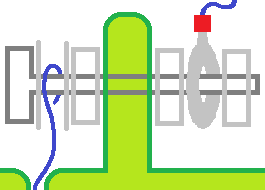
- two tubs with grid on four sides of each or shared center.
- fins for surface area.
- cages that snap in to the bottom of a tub shared by two cages.
- might use a part of a sphere for the snap in idea.
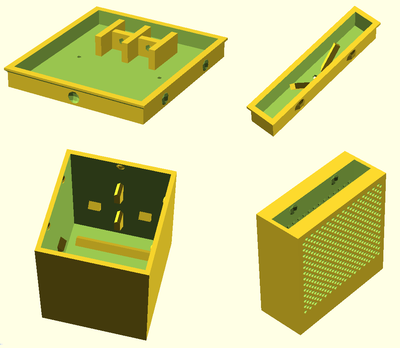
- tub lid
- tub lid will need some sort of two pole terminal block
- from cages below
- to next tub or out for use
- might need some sort of cover for uninsulated wires from cage
- vent for gas?
- tub lid will need some sort of two pole terminal block
- need to make parametric for 10 tub battery
- maybe input number of rows and columns so it can be a single row or two side by side
- might move side spacers onto cages. 3 sphere divots. 1 middle top and 2 side by side bottom, flip on opposite face. 1 or 2 on non mesh sides.
lessons learned
- 3d printing with layers most often leads to leaks
- can sometimes hold but not reliably.
- perhaps in the future print settings could be optimized
- need to use a regular plastic container for tub
- perhaps half gallon milk jug
- can sometimes hold but not reliably.
sketches
single cell separated by a grid.
File:BATTERY-idea-20141012.skp
scad work
parametric code to produce stl files for custom sized 3d prints.
User:Dorkmo/Ideas/Battery/SCAD
chemistry
KOH corrosion
- need to find out if ABS or PLA or other plastics will hold up to corrosion by the electrolyte
calculations
- nickel hydroxide Ni(OH)2
- 4.10 g/cm3
to research later
- would liked to have an info for:
- Ah/gram
- impact surface area has
- calculate AWG output based on dimensions of cage and cells
analysis
Need to find a battery analyzer.
bom
hardware
nickel, aluminum, brass?
- all thread
- bolts
- washers
- nuts
chemicals
wire
Misc
Notes
- internet sometimes suggests having 2:1 nickel hydroxide to iron ratio
- initial charge
- a few places have suggested that a short (30min) small charge happen 1 day before a full charge
- couldnt find any info on why this would be
mixes
- it is suggested to add graphite to nickel hydroxide
- internet says 17% graphite
- will try 85% nickel hydroxide 15% graphite to keep it simple
- one source said graphite is mixed into the iron
- havent seen that suggested anywhere else
- its also suggested that layers of nickel flake were used
- multiple layers were pressed with hydraulic force
- internet suggests 20-30% KOH in electrolyte
- tolerant of different amounts
version 1
- 1 cup dry Ni hydroxide powder
- 1/4 cup dry graphite powder
- then
- 1/4 cup black strap molases
- 3/16 cup water
FDM watertightness
http://www.stratasys.com/solutions/finishing-processes/sealing-fdm-parts
epoxy resin to seal surfaces
- instead use common LDPE containers?