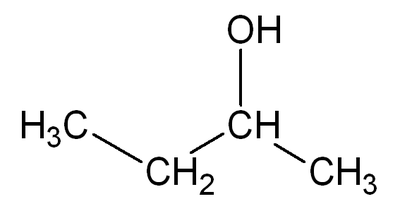
Butanol: Difference between revisions
Jump to navigation
Jump to search
(Added some more information) |
(Added some more links under the "External Links" section) |
||
| Line 28: | Line 28: | ||
*[http://en.wikipedia.org/wiki/Butanol The Wikipedia Page on Butanol] | *[http://en.wikipedia.org/wiki/Butanol The Wikipedia Page on Butanol] | ||
*[http://en.wikipedia.org/wiki/Acetone-butanol-ethanol_fermentation The Wikipedia Page on Acetone-butanol-ethanol fermentation] | *[http://en.wikipedia.org/wiki/Acetone-butanol-ethanol_fermentation The Wikipedia Page on Acetone-butanol-ethanol fermentation] | ||
*[https://en.wikipedia.org/wiki/Gevo,_Inc The Wikipedia Page on Gevo Inc] (Supposedly making this commercially viable (also not dead unlike 2/3 of these biofuel companies from the 2000's?) ( '''Make a page on them later''' ) | |||
*PESwiki: [http://peswiki.com/index.php/Directory:Butanol Butanol] | *PESwiki: [http://peswiki.com/index.php/Directory:Butanol Butanol] | ||
*Article: [http://tchie.uni.opole.pl/freeECE/S_18_1/Kaminski_18(S1).pdf BIOBUTANOL - PRODUCTION AND PURIFICATION METHODS] | *Article: [http://tchie.uni.opole.pl/freeECE/S_18_1/Kaminski_18(S1).pdf BIOBUTANOL - PRODUCTION AND PURIFICATION METHODS] | ||
Revision as of 07:57, 2 January 2021
Basics
- It can be used as a direct Gasoline Substitute in unmodified internal combustion engines
- Butanol is far less corrosive than ethanol and can be shipped and distributed through existing pipelines and filling stations
- It is an effective Hydrogen Carrier in a Hydrogen Economy ; Reformed butanol has four more hydrogen atoms than Ethanol, resulting in a higher energy density
- Butanol is an industrial commodity, with a 370 million gallons per year market with a selling price of $3.75 per gallon.
- It can be, but does not have to be, blended (10 to 100 percent) with any fossil fuel.
- Butanol can be transported through existing pipelines for distribution.
- It can be blended with petrodiesel and with vegetable oils (where it also reduces the gel temperature point and the viscosity) to produce biodiesel. ?
- It is a very versatile fuel and fuel extender in both gasoline and diesel engines. Octane?
Production Routes
Biological
Acetone-butanol-ethanol fermentation
- Typically done via Clostridium acetobutylicum
- Any genetically modified algae/e. coli/yeast etc?
- This can be done with a starch or sugar based feedstock
- It can also be done with syngas (See Syngas Fermentation )
Catalytic
Internal Links
External Links
- The Wikipedia Page on Butanol
- The Wikipedia Page on Acetone-butanol-ethanol fermentation
- The Wikipedia Page on Gevo Inc (Supposedly making this commercially viable (also not dead unlike 2/3 of these biofuel companies from the 2000's?) ( Make a page on them later )
- PESwiki: Butanol
- Article: BIOBUTANOL - PRODUCTION AND PURIFICATION METHODS
- Company: Green Biologics - a lot of proprietary stuff