Polyethylene from Ethanol: Difference between revisions
| Line 81: | Line 81: | ||
=== Polyethylene product characterization=== | === Polyethylene product characterization=== | ||
Polyethylene can be characterized according to physical material characteristics and molecular structure measurements. Shearing, density, flexibility are examples of easily measured attributes, while specialized tests are also employed and need to be reviewed. UV-visible light absorbance spectroscopy would give precise measurements of products suitability for greenhouse glazing (block UV, doesn't block blue and red). Measuring molecular structure is possible with various kinds of spectroscopy, especially advanced techniques such as NMR. While advanced equipment like this will probably not be available to OSE, the lessons of advanced study should be applied and correlated to available means. | |||
=== Value adding === | === Value adding === | ||
Revision as of 02:25, 16 May 2012
Main > Materials > Bioplastics
Introduction: Polyethylene
Polyethylene (PE) is a polymer of long chains of the monomer ethylene (IUPAC name "ethene"). It is one of the world’s most common plastics, with a wide range of uses and over 60 million tons produced worldwide every year. Several different categories exist, based on density and branching. Common types are high-density PE (HDPE; plastic # 2) and low-density PE (LDPE; plastic # 4). Polyethylene is not biodegradable, therefore significant environmental issues are associated with its use. Recycling of PE is relatively straightforward. When disposables are involved, every effort should be made to replace PE with biodegradable alternatives. However, resistance to biodegradation can also be a desired effect for some applications. For example, geomembranes are often made of HDPE and are widely used as liners for fish ponds, constructed wetlands and biogas digesters. Its resistance to degradation also warrants its use in the natural gas industry in transporting natural gas underground in high density PE pipes. Excellent chemical resistance of PE allows for widespread use in storage applications. PE is also useful as a material for digital fabrication. It can be used in the RepRap 3D printer.
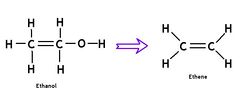
Polyethylene from ethanol two step conversion
Ethene is a very simple two-carbon organic molecule (C2H4) that does not have to be derived from petroleum. In fact, it can easily be produced from ethanol in a dehydration reaction. This has been known for many decades, but was not cost-competitive at low oil prices. Recently, a Brazilan-Japanese joint venture announced the "Green Polyethylene Project", with sugarcane as the feedstock. Commercial-scale introduction of this "BIO-polyethylene" is planned for 2011. We welcome PE to the club of bioplastics and believe that small-scale production from ethanol can be made practical.
Dehydration of ethanol seems fairly simple to do with an aluminum oxide catalyst. This method is well suited to small batches and could be easily scaled up to larger batch sizes. It sounds fairly easy to test out. They don't mention the required temperature but it has to be lower than the ignition point of ethanol(~362°C). If we want food-independent ethylene production, especially for larger scale use, we could go from carbon dioxide and water to syngas (a mixture of carbon monoxide and hydrogen) and then finally to ethylene [1]. This [2] may be useful for producing the syngas.using a fluid bed reactor or recently in a microreactor. The production of a distillation chamber capable of lowering pressure may also benefit the aluminum refining process. Aluminum is a favored catalyst for ethanol dehydration to ethylene but additional compounds such as transition metals increase yield and selectivity while other zeolite catalysts have also been described (Chen et al.).
Polymerization of ethylene is an exothermic reaction with multiple generations of catalysts. Phosphoric acid is the earliest catalyst under high pressure and temperature. Zeolite initially of silicates and then other matrices made the second generation of catalysts and still operated under elevated pressures and temperature. The third and currently evolving class of catalysts are known as Ziegler-Natta catalyst use an activator molecule of the (Al)C2H5n organoaluminum cocatalyst or methylaluminoxane and a titanium catalyst (TiCl3 or TiCl4 etc).
There are a number of steps involved in polyethylene production from a biotic feedstock; selection of a feedstock, construction of open source fermentors, purification equipment, and fluid bed reactors, along with methods of measuring yield and quality of each step will be require bringing a diverse background of knowledge together.
Status Brief
Almost all PE today is derived from petroleum. In a very energy-intensive process, a petroleum feedstock is cracked at high temperatures. After distillation and purification in large, capital-intensive facilities, ethylene is produced. This is then polymerized to polyethylene, a process that again involves high temperatures, high pressures and often toxic organic solvents. Clearly not an ideal situation.
An OSE project to replace this process with a constructive route from organic feedstocks rather than degradative oil based processes is currently in the research and development phase. A literature review below details the major steps of the process, technologies employed, and applicable details to an OSE process. Help with technical review and people with experience in the field is needed. A thorough proposal for a polymerization catalyst is an important missing step.
Documentation Brief
A thorough review of the process of creating polyethylene from ethanol is underway on this page. A catalyst protocol for the dehydration step is underway. A Systems Engineering Break Down is needed and an examination of the processes full product ecology. Assistance is needed summarize unreviewed literature and provide summaries of important information. A thorough review of the polymerization catalysts is needed.
Literature and patent review
Ethanol production
Ethanol can be produced on-site or purchased.
Ethanol to ethylene conversion
Ethanol’s hydroxyl group can be removed and replaced with a double bond via a dehydration reaction. The endothermic reaction is best catalyzed between 500-700 C and a reactor device capable of controlling the mixing of reaction reagents and catalysts under ideal conditions will be necessary to produce high quality ethylene capable of being polymerized. Steam has been used successfully to provide heat for the reaction and should by modular with the OSE steam generator and solar concentrator. http://www.cheresources.com/invision/topic/7179-ethylene-from-ethanol/
Fluid bed reactor allows easy interface solid catalyst and gas phase reagents and separation of gas phase products and has many advantages for alkene polymerization processes. Designs can incorporate a number of features for continuous use with features such as catalyst recycling, and constant input and output of substrate and product. Production of ethylene from ethanol and the polymerization of polyethylene from ethylene are carried out in fluid bed reactors and development of a multipurpose machine is proposed as part of the OSE product ecology. Information on the the design of FBRs and the first open source FBR purposed for plastic production should be collected on the Fluidized_bed_reactor wikipage.
Acids or metals may be used for catalysts for this reaction, however aluminum silcate is a catalyst that offers high yields and is easy to obtain and work with. AlO3 is a favored catalyst which will be produced by the soil aluminum extractor. However the addition of other compounds as either supporting material or doping of the catalyst has been found to increase yield and purity.
US patent 4,234,752 Dehydration of alcoholsissued to Wu et al. on November 18, 1980 details a method of using treated gamma-alumina as a catalyst for the dehydration of alcohols. The catalyst is base treated to remove excess surface acid sites which contribute to isomerization. An inert gas transports gaseous alcohol through the catalyst and minimizes side reactions. The described method has been found be effective with primary, secondary, and tertiary alcohols of 2 to 20 carbons with temperatures 200 to 500 C and pressures of 50 to 3500 kPa.
US patent 4,302,357, Catalyst for production of ethylene from ethanol, issued Nov 24, 1981 to Kojima et al. details a catalyst of high purity aluminum silcate of at least 99.6% purity with a phosphate of group IIa, IIb, IIIa, IVb in the wt% of .05 to 5 with a process for its preparation. Aluminum starting material that is capable of producing aluminum silcate under hydrolysis conditions should be utilized and higher purity organic aluminum salts or metallic aluminum is preferred. The primary factor influencing yield is purity of catalyst but pore volume and specific surface area also affect the reaction and ranges 0.15 to 0.50 cc/g and 100 to 350 m2/g respectively are recommended. Magnesium, calcium or zinc is recommended as a phosphate metal cation. The catalyst should be maintained at a temperature between 300 and 450 C.
Catalytic dehydration of bioethanol to ethylene over TiO2/g-Al2O3 catalysts in microchannel reactors by Chen et al. gives a general description of microreactors, catalyst configurations, and details a AlO3 catalyst with TiO2. Microreactors are small precision engineered devices to mix small reactions with a catalyst and heat. Microreactors may be a more suitable design for OSE specifications over traditional fluid bed reactors and were found to be more efficient than fixed bed reactors in this study. Chen et al found AlO3 doped with 10% wt TiO2 have high ethanol conversion efficiency, ethylene selectivity, and long-term stability of over 400 hrs. TiO2 increases that range of active lewis base configurations in the AlO3 matrix and enhances catalytic activity. Temperatures of 420+ C and ethanol concentration of 30-50% were found to be optimal.
Ethylene is a key plant hormone and is produced in "large" amounts by some ripening plants. Is there any feasibility in trying to capturing ripening fruits ethylene gas?
High purity ethylene product purification
Very high purity ethylene is required for polymerization, however processes for doing so are known in the art. Removing unreacted ethanol from the product stream by cooling below ethanol's point of 78 C and optimizing conditions for condensation removes low volatility molecules mainly water and ethanol. Breakdown and side products are mainly acetylaldehyde and more minorly ethane as reported by cheresource forums to be the side products of organic ethanol conversion to ethylene. Contamination such as higher complexity molecules, thiols, and other organic contaminants from the ethanol substrate can have unpredictable effects on the the reaction. Strategies to remove impurities from ethylene derived from hydrocarbon cracking have been the predominant focus of research and information on purification of organic ethanol derived ethylene is minimal. In industry fractionation columns used in tandem is a common strategy capable of producing very high purity ethylene suitable for polymerization. A process to dehydrate ethanol to ethylene can minimize the range and amount of contamination and should be a key design goal for the process. OSE may be benefited by pursuing strategies to produce high purity hydrous ethanol for use with a AlO3 and TiO2 under optimal conditions and a simple purification strategy that removes high volatility condensable gas products from what should be the majority product, ethylene.
http://www.google.com/patents?id=pfYsAAAAEBAJ
http://www.google.com/patents?id=EEQjAAAAEBAJ
http://www.google.com/patents?id=aVA_AAAAEBAJ
http://www.google.com/patents?id=jt1GAAAAEBAJ
http://www.google.com/patents?id=CJY1AAAAEBAJ
http://www.egr.uri.edu/che/Faculty/Lucia/Tutorials/tutorial2.html
Ethylene product measurements and quality control
Ethylene to polyethylene polymerization
The earliest used industrial catalyst for polymerization of olefins was supported phosphoric acid but were replaced with Ziegler-Natta catalysts in the 1960s.
Polymerization from ethylene to polyethylene takes controlled high temperatures and pressures, and requires the precise control of the movements of reagents. A reactor chamber capable of processing ethylene to polyethylene without fouling will be required. Catalysts capable of polymerizing oleofins with high efficiency and selectivity for long-chain, with low branching, and high crystallinity have been in use for many decades and much information on their use is freely available.
US Patent 4,003,712 issued to Miller on Jan. 18, 1977 details a catalyst of silyl chromate for olefin polymerization with high reactivity and fluid bed reactor for its use. The reactivity of the catalyst allows small enough quantities to be used to avoid product fouling. The catalyst can be supported with a number of materials to enhance its action and extend its use.
US patent 4,383,095 issued to Goeke et al on May 10 1983 details a catalyst made of magnesium and titanium which is capable of polymerizing high density polyethylene polymers in a fluid bed reactor that display many favorable characteristics for casting and injection molding. The titanium compound consists of the element with a hydrocarbon free radical and halogen. Magnesium halogen salts are preferred with anhydrous MgCl2 being favored. The catalyst is supported on a bed of inert porous material such as silica. Density of the resulting polymer is controlled by the addition of aluminum paired radical hydrocarbon olefins electron donors of C3-C8 for copolymerization, this is added directly to the reaction mix in the order of less than 10% total olefin substrate volume. The patent details a preparation procedure to impregnate the support material with the titanium and magnesium catalysts and preparations for the direct addition of the activator compound to the reaction. Discussion of the fluid bed reactor can be found in the fluid bed reactor for plastic synthesis literature review.
http://www.google.com/patents?id=cupVAAAAEBAJ US patent 2,395,381 issued to Squires on Feb 19, 1943 details methods to polymerize ethylene with vinyl acetate using oxygen or more preferably peroxy catalysts under high pressure and elevated temperatures.
http://www.google.com/patents?id=5j5-AAAAEBAJ
A simple version of the Ziegler-Natta catalyst seems feasible for OSE and can utilize the same elements (Al and Ti) as the dehydration step. Triethylaluminium (TEA) is an organoaluminium activator cocatalyst that is available in commodity form and has a relatively straight forward synthesis. Purchased cocatalyst combined with titanium and/or magnesium, possibly on a silicate matrix could be prepared on a small scale and used in an polyethylene polymerization.
Polyethylene product characterization
Polyethylene can be characterized according to physical material characteristics and molecular structure measurements. Shearing, density, flexibility are examples of easily measured attributes, while specialized tests are also employed and need to be reviewed. UV-visible light absorbance spectroscopy would give precise measurements of products suitability for greenhouse glazing (block UV, doesn't block blue and red). Measuring molecular structure is possible with various kinds of spectroscopy, especially advanced techniques such as NMR. While advanced equipment like this will probably not be available to OSE, the lessons of advanced study should be applied and correlated to available means.
Value adding
Polyethylene recycling
Proposal of action
Producing polyethylene from locally produced base materials and open source hardware will require the production of high purity molecules and machines capable of conversion at high efficiency and selectivity. The project can be broken down the three aims of producing high quality ethanol, ethylene, and polyethylene. The tasks need to be further divided into catalyst selection, hardware components, and substrate requirements to be worked on separately as part of the scrum process. Task 2, the dehydration of ethanol to ethylene, will be the first goal of the project as it has the largest value margin between substrate and product and the catalyst requirements are within the scope of OSE's currently proposed product ecology.
1. Production of ethanol on-site from sorghum utilizing yeast fermentation. A. Selection of yeast and/or bacterial strains that are optimal for sorghum fermentation and finding their optimal conditions. B. Construction of fermentation equipment. C. Construction of distillation equipment capable of operating under vacuum, which could possibly be attached to fermentation chamber. D. Method for measuring alcohol purity. Measuring specific gravity is means of getting a rough estimating ethanol yield and with internal improvements can achieve higher accuracy. Measurements against as internal standard and a pure ethanol standard can improve hydrometers accuracy.
2. Dehydration of ethanol using a catalyst and fluid bed reactor. A. Selecting a catalyst. AlO3 can be utilized as an initial catalyst after production by the aluminium extractor. A base wash with KOH or NaOH can be used to increase the specificity of catalyst. Improvements to the catalyst can be incrementally made as OSE technology becomes available. B. Constructing a reactor chamber capable of mixing the catalyst and substrates under optimal conditions. Reactor chamber must allow control over temperature, pressure, addition and removal of catalyst, control of feedrate and interaction time of substrate, and separation of production and should incorporate features that allow easy reconfiguration and recycling of catalysts, solvents, and unconverted substrate. C. A fractionation column will be used to remove byproducts, unreacted substrate, and inert gas, producing high purity ethylene suitable for polymerization. C. Measurement of ethylene yield and purity using spectroscopic methods.
3. Polymerization of polyethylene from ethylene using transition metal catalyst and fluid bed reactor. A. Selection of a catalyst for polymerization. B. Optimal configuration of reactor for polyethylene polymerization. C. Measurement of PE yield and purity. D. Ability to pass newly formed polyethylene to an extruder or storing as pellets for future extrusion. E. Investigate production of other polymers such as polyethylene vinyl acetate (for greenhouse materials).
4.Extrusion to final product A. Identify most desirable products for OSE product ecology and research optimal extrusion processes. Materials for greenhouses or windows are a high priority as mentioned by Marcin and this application could be the first aim. Identify ways to maximize translucence, increase UV resistance and filtering, and maximize material use with strength and durability (film versus panels) B. Value adding processes such as tensile polymer incorporation or shaping into useful products.