Lime
Jump to navigation
Jump to search


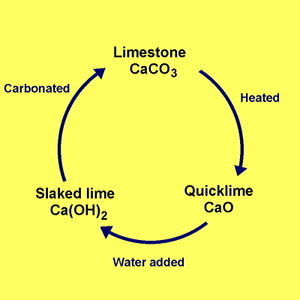
Image from Youtube video: "Limestone Cycle - limestone, quicklime and slaked lime" (Chemistry for All - The Fuse School)
Basics
Lime is an extremely versatile basic material. Limestone, often composed largely of calcium carbonate (CaCO3), can be burned in a kiln. When heated to 900-1000C for several hours, it vents off carbon dioxide (CO2). What remains is mostly calcium oxide (CaO), also known as “quicklime” or “burnt lime”, a highly caustic material that is very “thirsty” for water. When combined with water – hydrated or “slaked” - the quicklime becomes calcium hydroxide or Ca(OH)2, often simply referred to as "lime". This material quickly reabsorbs CO2 and once again becomes calcium carbonate, completing the lime cycle.
Historical uses for hydrated lime:
- mortar for construction
- agriculture: to neutralize acidic soils to crop production
- "whitewash" - to protect wood (such as fences) or fruit trees from fungal infections
- as a disinfectant: water treatment, dairy, as an antiseptic for livestock
Historical uses for quicklime:
- main industrial uses today: as a steel fluxing agent and in flue gas desulphurization. Other: production of fiberglass, pulp and paper, aluminium, uranium, copper and gold.
Product ecology
- as a stabilizer in compressed earth blocks
- for mixing with hemp, to make "hempcrete"
- to fire the kiln, one could use the pyrolysis off-gases from biochar production (see: Biochar-Lime Co-production System)
- use waste heat (from burning and from slaking) to heat greenhouses, fish tanks, other facilities
Links:
- Low-Tech Magazine:"Burning the Bones of the Earth: Lime Kilns"
- Wikipedia: Lime (material)
- Practical Action: "Lime - an introduction"
- Practical Action: "Lime - Kiln Designs"
- Practical Action: "A Small Lime Kiln for Batch and Continuous Firing"
- Practical Action: "How to Build a Small Vertical Shaft Lime Kiln"
- Nice report showing the comprehensive process, and batch + continuous kiln design descriptions - [1]. Source: GATE -