Fermentation: Difference between revisions
m (→Related Wiki Pages: internal links) |
|||
| Line 41: | Line 41: | ||
==Related Wiki Pages== | ==Related Wiki Pages== | ||
*[[Fermentor]] | |||
*[[Silage]] | *[[Silage]] | ||
*[[Prebiotics]] | |||
*[[Biorefinery]] | |||
*[[Acetone-butanol-ethanol fermentation]] | *[[Acetone-butanol-ethanol fermentation]] | ||
*[[ | *[[Biogas]] | ||
==External Pages== | ==External Pages== | ||
Revision as of 23:04, 20 June 2016
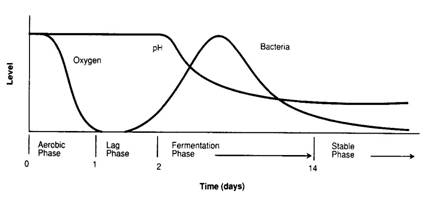
The second or anaerobic phase begins when available oxygen is used up by respiration and aerobic bacteria cease to function. Anaerobic bacteria (bacteria that grow in the absence of oxygen) then begin to multiply rapidly and the fermentation process begins.
The best silage is produced when the most rapidly growing microorganisms are predominately of the lactobacilli species, as they produce lactic acid from the fermented plant material. Lactic acid lowers the pH of the silage. Fermentation completely ceases after three to four weeks when the pH becomes so low that all microbial growth is inhibited.
(Source: Nutritional Ecology of the Ruminant, P. Van Soest, Cornell University Press, 1994, p. 217.)
Fermentation is a metabolic process where organisms use an alternative to oxygen as a final electron acceptor in order to gain energy. Often the same molecule from which high energy electrons were harvested ultimately accepts the lower energy electron in a different molecular configuration, an example is the fermentation of glucose to ethanol. Fermentation by microorganisms has been mastered by humans for millennia, first for food preservation and more modernly as a source of chemicals.
Fermentation is a process that must be conducted free from air and atmospheric oxygen, and can be conducted either submerged in water (liquid state) or in a seal container. Depending upon the starting material and the sugars it contains and the product desired a variety of microorganisms can be selected.
Food preservation
Fermentation will be used to preserve different food sources for longer term storage. Fermentation acts to preserve food by converting universal energy source sugars to fermentation products which cannot be used by all microorganisms. Secondly the fermentation products create an environment that is not conducive to other microorganisms, mainly by lowering the pH. The human gut flora contains many of the same microorganisms used in fermentative food preservation and consumption of fermented foods can have a high impact in improving digestive health and function.
Examples:
- Yogurt, Kefir
- Sauerkraut, Kimchi
- Silage for livestock
- Kombucha
- Beer
- Cheese
Sauerkraut juice as starter
Sauerkraut juice is especially rich in lactofermenting organisms. It can therefore be used as a starter for other lactofermentative processes. It can also help with microbiome maintenance on the farm. By controlling the microbial composition (pigsty, cattle housing, chicken coop, etc.) beneficial effects can be achieved, such as reduced risk of antibiotic resistance, better animal health (reduced risk of infection), suppression of bad odors, possibly beneficial effects on feed efficiency, etc. This topic requires a dedicated page.
Fermenting Chicken Feed
Fermenting chicken feed may have the following advantages: improved bioavailability of nutrients, higher feed efficiency (hence, lower cost), better intestinal microbiome of the animals.
Article from Permaculture Research Institute: "Cut Your Chickens Feed Bill by Fermenting".
Chemical Engineering
Fermentation products include small organic molecules with chemical functional groups that are useful for chemical engineering. Fermentation for chemical production has been commercialized and showed promise in the early 1900s before the refinement of the petrochemical industry.
Examples:
- Ethanol
- Butanol
- Acetone
- Lactic acid
Related Wiki Pages
External Pages
- No Tech Magazine: "Preserving Food by Fermentation"
- Wikipedia: Lactic acid fermentation