Pyrolysis Oil
Basics
Pyrolysis Oil or "bio-oil" is a product of Pyrolysis of various materials such as:
- Upon heating of wood or other biomass to sufficient temperature in a low/no oxygen enviroment, volatile components are produced
- After cooling, some of these are gaseous (hydrogen, carbon monoxide, gaseous hydrocarbons) while others are in liquid form, the so-called pyrolysis oil
- This oil is a dense fuel source - for applications such as heating and steam production
- It may be burned by a burner such as the Babington burner
- As such, this is a lower-tech substitute for petroleum fuels in some applications, with lower caloric value than diesel fuel.
- At present, it cannot be substituted for diesel in standard diesel internal-combustion engines due to high viscosity and acidity
- Upgrading of bio-oil to Diesel fuel via the Fischer-Tropsch process is possible but may not be practical small scale
- Chemical Pathways Also Exist
- Recently, a cheap and open source way to upgrade bio-oil via Red Mud as a catalyst was discovered
Hazards
- Need to find some sort of MSDS sheet for it, or a similar product "creosite" / wood tar perhaps?
- PROBABLY not too good
- Can't hurt to use:
- Gloves
- Fume hood, and respirator and/or good ventilation in the workspace
- Until it is turned into the final fuels/products, then just use:
- Good Ventilation, and wash hands after exposure
Pyrolysis Oil Applications
Use Cases
Pyrolysis oil is most often derived from biomass pyrolysis, but a wide variety of other sources are also possible, such as waste plastic and old tires. Typical industrial applications of pyrolysis oil as a fuel:
- Boilers
- Furnaces
- Hot Water Generators
- Hot Air Generators
- Thermic Fluid Heater
- Electric Generators (mixed with 50% diesel)
- Diesel Pumps (mixed with 50% diesel)
Methods of Use
- Can be Directly (albeit not as efficient and more polluting) Used As:
- Waste Oil Fuel for use in Waste Oil Burners
- Oil Lamp Fuel for non-electric lighting
- Can it be a lubricating oil, especially for machining?
- If processed it can be used as the respective hydrocarbons
- Filtration, Water Seperation + Chemical Drying, and Fractional Distillation is the main workflow
- Can it be used as a feedstock for Bio-Diesel Production ?
- The reactors will most likely also produce these other usable products:
Workflow
Production
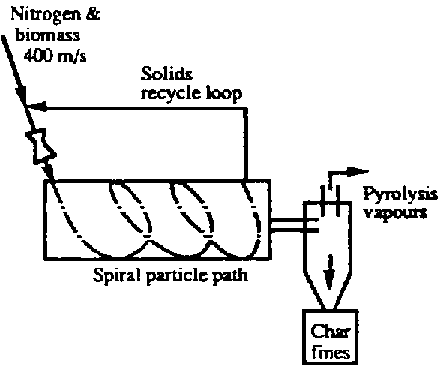
- Pyrolysis Oil Can be Produced in a Number of Ways:
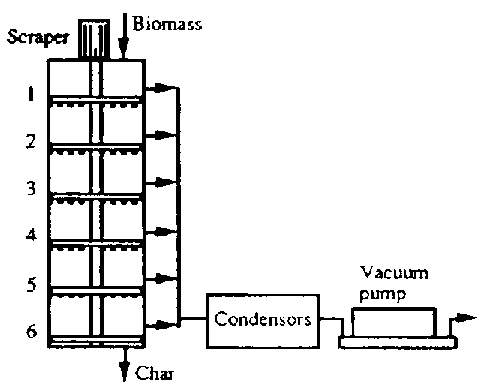
- Pyrolysis Kilns can, as with any reactor, be operated as a Batch Process or as a Continuous Process
- They can also be Fixed Bed or Fluidized Bed
Post-Production Filtration
- Simple Vaccum Filtration through a filter
- May even be able to use a fine metal mesh for re-use (acidity may be an issue for this however, perhaps fabric/ceramic?)
Water Seperation + Drying
Basic Seperation
- A Seperatory Funnel or similar device (Pipet method for small volumes, buckets with pour spouts for cheap setups, etc) can seperate most of the water
- Can a diesel-water seperator/trap be a good OTS option?
- An automated batch device, or a Continous Oil-Water Seperator may be of use
Further Drying
- This is not needed for direct use
- This is more so for use in chemical refinement and upgrading
- This can be done via:
- Molecular Seives
- Vacuum Drying (assuming oil wouldn't boil off first, or this is taken into account)
- Fractional Freezing (Freeze Drying of liquid mixtures for seperation via sublimation differences) may work (needs research)
- Simple Evaporation Dishes/Ponds in a dry/warm enviroment? (needs more research)
- Can addition of ethanol, then distillation of the Azeotrope off (at a temperature below the minimum boiling point of the Pyrolysis Oil) work well?
Post-Filtration+Drying Refining/Upgrading
- Not used for any direct oil use cases
- This is used to make synthetic hydrocarbons on the same level as Bio-Crude or Non-Renewable Crude Oil based varieties
- Done via the same workflow as the other sources:
- Fractional Distillation
- other petrochemical processes (upgrading and all that catalyst stuff if needed/desired)
Student Projects
A great experiment that can be done in a semester is the construction of a simple distillation apparatus to test the procedure with wood chips or newspaper - and to measure the purity and composition of the resulting fuel. Contact: joseph.dolittle at gmail dot com for more information
Basic Experiment
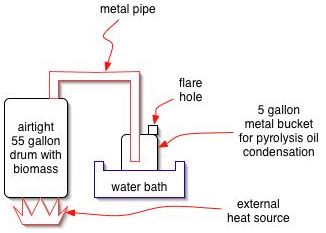
A basic experiment can be performed readily by heating biomass in a 55 gallon metal drum. An external fire may be applied, or an electric heating element may be installed. The vapors that come off can be sent into another drum, submerged in cool water for condensation to occur. A weep hole is put in the second drum for pressure release, and gases may be flared, or captured, at this outlet as the reaction proceeds. When all biomass is distilled, the flare gassing will stop.
The resulting product can then be analyzed.
- Flammability test
- Is fuel readily usable in a Babington burner?
- Heating to drive off water
- Heating to drive off lighter fractions to produce fuel oil
- Further heating to provide heavier oils or lubricants
- Cooling to separate phases
- Freezing to separate phases or to separate water
- Using a water jug with an integrated tap allows for easy water fuel seperation (similar to a seperatory funnel)
- A Paint Can as a reactor may be even easier to do small scale, and cheaply
More Information
Comment from Elliot Hallmark:
here's a pyrolysis machine as i understand it:
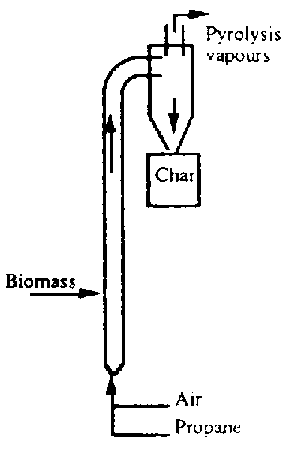
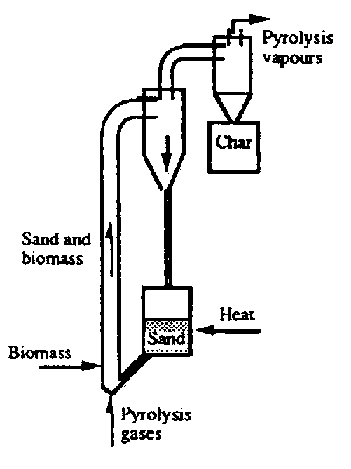
1. you need a furnace, probably an old barrel for the outside of the combustion chamber lined with with a fireclay/sand/sawdust mixture inside. It would have a lid with a moderate exaust port (maybe half the area of the lid is removed), which could be cast out of the same fireclay mixture. Also, there's an opening at the bottom for fuel and air. You could run it on natural gas, since eventually you'd probably just pipe the wood gas back into it in a later version.
2. a chamber for the to be pyrolyzed material to go into. might be able to surround a cheap chamber with a thin protective coating. thin so as not to impede heat transfer. refractory mortar and sand maybe, the mortar is maybe $20 for all you'd need i think. or you may need to use a large diameter pipe and make a bottom and top for it out of thick (5/8"-1/2" i guess) metal slabs. it has to be somewhat thick because it will oxidize through quickly otherwise (galvanization would evaporate; chromium or enamel would have to survive thermal expansion/contraction cycles; thin stainless may be an option) at the top there is a hole for outlet, there is no inlet hole.
3. a quencher. apparently the quickness of the quenching is important, as the free radicals produced are rapidly combining to form tar and asphault as opposed to more useful things. A common way of doing this is to spray a lot of cooled pyrolysis oil into the hot stream within a cyclone seperator (like your flour mill thing). I don't know how practical that is. perhaps cooling the walls of the cyclone seperator and the tubing up to it also with running water from your cold well would work. this woukld need experimentation.
4. gas storage. an oil drum filled with water, inverted and subersed in water. A large version of how they collect gas in chemistry class. bubble the gas in through the bottom and you've got a valve on the exposed surface to let the gas out at your leisure. weights on top of the barral determine the psi of the storage. eventually this gas might be just redirected back to the furnace, but at first its good to know how much gas you are getting, and also you could use it as cooking gas to displace your propane.
At first i say skip 3 and just let bubbling through the water in 4 be the quench. then you could weigh the char and the gas and know how much oil you are producing. most of the oil would probably be in a film at the bottom of the gas collection, but i dont know how the wetness would affect it (i think some fractions would polymerize with the water, or form a stable emulsion). Theoretically, this would be the best quench as far as surface area of gas to thermal sink goes, so you'd get an estimate of how much oil very effective quenching would produce. then, when you have system data on flow rates and all that you can build a cyclone seperator and play with some better quenching ideas.
-elliot
Prototype 00 Simple Drawings
Video: distillation of wood-based bio-oil
This video by Youtuber Mr. Teslonian demonstrates the small scale fractional distillation of bio-oil from a wood stove. Very clean gas is produced by running it through a micro-refinery (with filters and fractional distillation system). After distillation, the gas powers an internal combustion engine which runs an electric generator.
Internal Links
- Pyrolysis Oil Handling Precautions
- Pyrolysis Oil Refining
- Pyrolysis
- Open Source Fuel Agnostic Pyrolysis Batch Kiln
- Fluidized bed reactor
- Biofuels
- Babington Burner
- Biochemicals from Pyrolysis
- Negligiblek's Pyrolysis Empirical Model
- Bioasphalt
- Biocrude
- NatureJab
Case Studies
- Plant built in Pakistan for landfill plastic waste - [1]. Only $200k capital cost for millions of revenue? This appears to be a study only, but if plant cost is low and garbage is used, this could work. Looks like their numbers make no sense.
DIY Experiments
- Living Web Farms - [2]
Yields
- 30% diesel and gas from poplar by weight with 7% moisture - USA DoE - [3]
External Links
- Pyrolysis Oils: Characterization, Stability Analysis, and Catalytic Upgrading to Fuels and Chemicals
- Interesting article on the pros cons and problems and uses of pyrolysis oil.
- The cons mentioned - are pros for Factor e Farm if we like biochar and external combustion engine fuel. - MJ
- RESEM Pyrolysis Plant A Leading Pyrolysis Plant Manufacturer
- PyNe - The Biomass Pyrolysis Network - a global network of active researchers and developers of fast pyrolysis, has been established to discuss and exchange information on scientific and technological developments on pyrolysis and related technologies for the production of liquid fuels, electricity and chemicals. Interesting table on the various types of reactors and their relative value to difficulty of construction and quality of fuels. table at bottom
- Paper: "An exploratory study on the removal of acetic and formic acids from bio-oil" Media:BioRes 04 4 1319 Sukhbaatar SIK Expl Removal Acetic Formic Acids Bio Oil 450.pdf
- Cornell U. project: "Village-scale pyrolysis for liquid biofuels" (Biofuels and biochar production for energy self-sufficiency and agricultural sustainability)
- "The Future of Bioenergy Is in this Book-less Library"
- Another Paper
- A Video on Plastic to Pyrolysis Oil
- A 2010 Paper Titled "A high-yield biomass alternative to petroleum for industrial chemicals"
- A 2017 Paper Titled "CONTINOUS SEPARATION OF COMMODITY CHEMICALS FROM PARTIALLY DEOXYGENATED PYROLYSIS OILS VIA FLASH DISTILLATION"
- A Video by the YouTube Channel "Advanced Materials Congress Lectures" Titled "Catalytic microwave-assisted pyrolysis for chemical recycling of plastic waste" ( ~20 Minute Watch )
- Add paper link here
- A Report on a 1994 Conference Titled “Biomass Pyrolysis Oil Properties and Combustion”
- A 2018 Paper in the Journal “Biomass and Bioenergy” Titled “The use of a fast pyrolysis oil – Ethanol blend in diesel engines for chp applications”
- A 2023 Paper Titled “Biomass pyrolysis oil/diesel blends for a small agricultural engine”