|
|
| (216 intermediate revisions by 17 users not shown) |
| Line 1: |
Line 1: |
| {{under construction}} | | {{OrigLang}} |
| http://en.wikipedia.org/wiki/Nickel_iron_battery
| |
|
| |
|
| ==Basic Concepts Behind Construction==
| | {{GVCS Header}} |
| The electrochemistry of a Nickel iron battery is similar to a NiCd or NiMH battery in that nickel oxyhydroxide is used as a cathode, but iron is used instead of the toxic metal complexes in NiCd and most NiMH batteries as the anode. During discharge, both metals turn into their hydroxide forms: Ni(OH)2 and Fe(OH)2. (see the wikipedia article under electrochemistry). It should be possible to build it in a discharged state, combining the appropriate hydroxides of Nickel and Iron. Alternatively, the battery could be constructed out of metallic nickel and iron, and the nickel could be converted to NiOOH or Ni(OH)2 in situ through oxidization with ozone or peroxide and UV or some other means. (We will definitely need someone who knows some chemistry and is willing to put in some time at at least several stages in this development process.)
| |
|
| |
|
| ==First test== | | =Overview= |
| A sample concrete cell was tested, but no practical benefit is achieved using concrete as an electrolyte, as to be effective, the entire cell must be submerged, and the electrical conductivity of cement is far too high when submerged. A new design is in the works for a cell which would use raw glycerine (glycerol) as a byproduct of biodiesel production, thus improving the ecologies of both systems. The rationale is that the lye (KOH or NaOH) which 'contaminates' the glycerine should prove to be an effective electrolyte, and the glycerine itself should support a more stable cell. (Glycerine will evaporate much more slowly than water, and due to higher viscosity, should even further improve vibration-resistance of the cell. Ideally, this design could even be adapted to portable units.
| | [[File:Nickel-Iron Batterypic.png|thumb|400px|Nickel Iron Battery]] |
|
| |
|
| ==Proposed prototype==
| | The '''Nickel Iron Battery is the only known lifetime design battery. These last 100 years, such as the Edison batteries unearthed after a century that work like new. Thus, it is the primary electrical energy storage device for the [[GVCS]], outside of indirect sources such as [[Compressed Air Energy Storage]], water [[Gravity Storage]], and storage of energy via [[Hydrogen Production]]. |
| Rather than mess too much with potentially difficult electrode geometries (sintered, meshes, 'cloth') I propose a simple solution which fits quite nicely into the project ecology. Please excuse the horrendous sketch, as I've not developed any cad skills yet, but here is a suggested design which calls for sheet steel, bar-stock, some kind of container, and nickel hydroxide to build a discharged cell. This design makes use of the cnc plasma cutter to produce a set of thin 'tabbed' plates for the battery. Relatively high current density is achieved by using thinner plates, and the limited current delivery serves as a self-protection mechanism. Sheets are cut to resemble the profile of a tabbed business folder, then every other sheet is flipped. The encasing for the battery will contain grooves along the sides into which the plates will slide. Holes will be cut into the plates to both modestly increase surface area and allow the electrolyte solution to move and conduct throughout the cell. Once assembled, the cell should have two rows of raised 'tabs' to each of which will be welded a piece of barstock to provide a common, high-current rail. Note, neither copper nor aluminum is used here in an effort to limit galvanic corrosion outside the cell. 10 of these cells should be arranged into two rows of five, and connected in series to achieve a single 12V battery.
| |
|
| |
|
| The reason these relatively difficult to make electrode geometries are used is in pursuit of higher active material utilization (major impact on cost) and higher discharge rates, mostly.
| | ==Advantages== |
| | *'''Theoretically unlimited lifetime''': Long lifetime of 8-10 years - when you don't throw out the battery - just replace the electrolyte. [http://www.s4solar.co.nz/information/products/nickel_iron_battery/] |
| | *About X = $1000/kW - but unlimited life means that the full-cost accounted cost means that the cost is really X/N - where N is a number that you choose. Think of this - the core lives for ever - you replace the casing and electrolyte. |
| | *Open source design of electrodes |
| | *Cells can be made to any Ahr rating |
| | *Nickel and iron obtained from scrap stream, reprocessed via [[Induction Furnace]] |
| | *Completely closed loop material cycle ecology |
| | *Max discharge/charge = C/2. Optimal charge/discharge - C/4. [https://ironedison.com/images/products/Iron%20Edison/NiFe%20Industrial/Iron_Edison_Nickel_Iron_battery_spec_sheet_2017.pdf] |
| | *Vidoe showing build of a simple cell - [https://www.youtube.com/watch?v=K84PywMwjZg] |
| | *Discharge at 1C rate appears to get 70% of battery capacity? Not likely, it must be more like time - [[File:1cnickeliron.png|300px]]. Source - [https://www.alibaba.com/product-detail/Max-long-life-nife-nickel-iron_1986518612.html] |
|
| |
|
| It is possible to make a battery from active nickel oxyhydroxide particles of 200 mesh that is rated at 0.1 C or so, which is about 0.075 mm. But if you have a surface(backed by metal) then the ratio of volume/area is T^3/T^2=T, where T is the thickness, whereas with a sphere it is (pi*d^3/6)/(pi*d^2)=d/6 where d is the diameter, thickness of the layer would have to be therefore 1/6th of 0.075 mm to have the same volume/surface area with a plate as a particle, or 0.0125 mm, 12.5 microns. If you use both sides of the plate then you get 0.0025cm*1cm^2=1/4000 cm^3, density of nickel hydroxide is 4 g per cc, so the weight of material per sq cm of plate is 1 milligram. You would get 10 grams per square meter of plate, which could be increased slightly (depending on plate thickness) with perforations, and you could make the plate foil 0.01 mm thick, but you still would need a lot of foil. It would have to be packed together in some way, and plating it with nickel would be that much more work.
| | ==Disadvantages== |
| | *The historical NiFe technology’s notable limitations include low specific energy, |
| | low power, low charge retention and poor low temperature performance along with being high |
| | cost1 and is still in limited mass production throughout the world today for specific applications. |
| | NiFe battery chemistry is known for its robustness, extreme shelf and cycle life. The historical |
| | NiFe technology that was most robust to abuse also had limitations in being heavy, low power, |
| | low charge retention and poor low temperature performance along with being high cost. Thus, |
| | over the years, other nickel battery technologies (e.g., NiCd, NiMH, NiZn, and NiH2) have |
| | displaced NiFe in many applications. [https://www.osti.gov/biblio/1323594-selected-test-results-from-encell-technology-nickel-iron-battery]. This paper discusses the 'improved' Encell cell with much shorter lifetime. |
| | *OSE Assessment: |
| | **Low specific energy - still perfect for stationary applications of renewable energy |
| | **Low power - in a renewable energy scenario, large loads are run from PV when the sun is shining. Battery storage is only carry-over through the night for essential activity such as computers and internet - not heating or cooling (high-priority overnight cooling needs, such as refrigeration, can still be met by Ni-Fe using devices with an [https://en.wikipedia.org/wiki/Inverter_compressor inverter compressors]). Thus, low power is not an issue. |
| | **Low temperature performance - is an issue if batteries are outside in freezing temperatures. |
| | **High cost - that is not an accurate assessment. They have a higher up-front cost, but their lifetime cost is significantly lower. Lead acid survives because it is a good car starter battery, but for power storage, it does not do well. |
| | **C/2 max discharge rate. |
|
| |
|
| Also that 200 mesh is with cobalt additive to improve conductivity which could change things a lot. Without it the layer would have to be thinner to get the same internal resistance. How much needs to be verified by finding the conductivity of the material with and without additive. Secondly those documents listed below will provide information about exactly what sort of surface area to volume ratio leads to what sort of battery characteristics, so we can decide if a higher v/a ratio may be acceptable.
| | =The Latest= |
| | *2012 - '''Ultra Nickel Iron Bats sighted in Nature''' - high performance nanotech batteries have been shown to be 1000x more powerful than Edison's originals and nearly 100% [[Coulombic Efficiency]] - in 2012 - [https://www.nature.com/articles/ncomms1921]. Thus, the potential is there for a safe and durable battery - even in automotive applications. |
| | *2020 - '''Batolyzer - Combination battery and electrolyzer'''- has been demonstrated for producing hydrogen in addition to storing energy, thus killing 3 birds with one stone. Energy storage, hydrogen production, and [[SDG 7]]. See paper at [https://www.frontiersin.org/articles/10.3389/fenrg.2020.509052/full] |
| | *2017 - High discharge, sintereed iron electrodes, 2017 - '''3500 cycles at 100% depth of discharge'''. Potential for solving grid storage. [https://iopscience.iop.org/article/10.1149/2.1161702jes/pdf] |
|
| |
|
| Also, there is no need for a self protection mechanism, as you call it, and you can't have high current density and and current limiting at the same time. Lower internal resistance is always good, within the range that is attainable.
| | =Detailed Description= |
|
| |
|
| Lastly the nickel hydroxide would fall off the electrode, unless held in place in some way. With a foam or other porous mesh-like electrode that is overcome with the mechanical support that the matrix provides.
| | The '''nickel-iron battery''' (NiFe battery) or "edison cell" is a storage battery having a nickel oxide-hydroxide cathode and an iron anode, with an electrolyte of potassium hydroxide (lye can be used as a substitute). |
|
| |
|
| I hope this helps with understanding for others who may find this page in the future as well.
| | The active materials are held in nickel-plated steel tubes or perforated pockets. |
|
| |
|
| [[Image:nife_childsplay.png]]
| | It is a very robust battery which is tolerant of abuse, (overcharge, overdischarge, and short-circuiting) and can have very long life even if so treated. |
|
| |
|
| ==Preliminary Figures for a 12V, 1kWh pile==
| | It is often used in backup situations where it can be continuously charged and can last for more than 20 years. |
| NiFe cells produce a working potential of 1.2V, and charge at 1.4V. A 12V battery would then consist of 10 cells, and charge at 14V which is typical for most "12V" batteries. To achieve 1kWh capacity, we will need 1000W/12V = ~85Ah. This means that each cell will need to provide 85Ah capacity. This corresponds to 306,000C, which is approximately 3.17 moles of electrons. Sheet steel will form a base material for the electrodes, so iron is not a limiting factor. Nickel's electrochemistry in an NiFe battery indicates a 1:1 molar ratio, so 3.17 moles of nickel will be required. This is about 185g of Ni at a molar mass of 58.69g/mole. For a 'safety' margin, we will round up to 200g. At 200g Ni per cell, a total of 2kg of nickel will be needed for a 1kWh unit. The actual capacity of this cell based on the rounded values above would be 1095.8Wh.
| |
|
| |
|
| ==Environmental Aspects==
| | '''Nickel-iron batteries''' have ~50 year lifetimes, compared to a few-year lifetime of lead acid batteries. They are environmentally more benign, and lend themselves to local recycling and fabrication. They can have higher discharge rates and faster charge times than lead-acid batteries depending on mechanical design of the electrodes etc, so they lend themselves not only to off-grid power, but also to power electronics applications such as welding and heavy workshop power. In China a company by the name of changhong batteries makes a version of them for use in automotive starter batteries. Their energy density is half that of lead-acid batteries, but their long lifetime and deep discharge ability makes them highly relevant to the [[GVCS]], including to electric farming equipment as the next generation of [[LifeTrac]] infrastructure. |
|
| |
|
| All batteries (at least the ones in common use) are toxic to a greater or lesser extent. The NiFe Battery isn't an exception, but it is far less toxic than Cadmium-based batteries.
| | The [[Edison Battery]] was developed and promoted primarily by Thomas A Edison. |
|
| |
|
| * Iron is non-toxic and commonly available.
| | =Product Ecology= |
| * Nickel Oxide is toxic. The Appropriate Technology Collaborative is investigating toxicity [http://apptechdesign.org/].
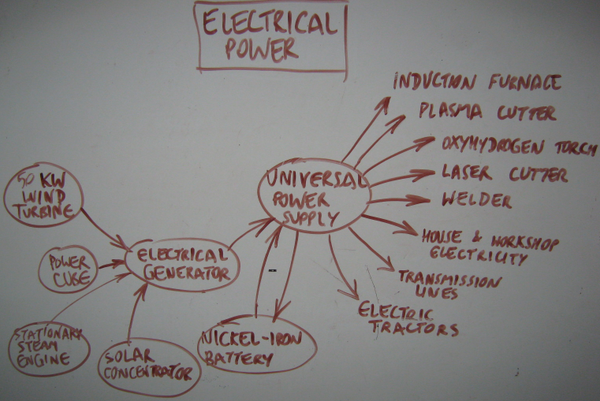
| | [[Image:Electricalpowereco.png|600px|thumb|[[Product Ecology]]]] |
| * The lye electrolyte is caustic and corrosive, but perhaps could be used in small amounts.
| |
| * Suspending the lye in glycerin also mitigates effects.
| |
| * Glycerin is a by-product of creating bio-diesel, thus using a waste of a different process.
| |
|
| |
|
| ==Sources and pricing==
| | {{Product Ecology |
|
| |
|
| FusionBeads [http://www.fusionbeads.com/shop/product/48723/] 3"x3" 24 gauge nickel sheet is $3.25.<br>
| | |Product={{Battery}} |
| Metric: 76.2x76.2mm and 0.5mm thick. The density of nickel is 8.902 g/cm3. 2903.22 mm3 which is 2.90 cm3. Thus, each sheet weights 25.84g. 2000g (2kg required as above) is 77.38 sheets. $251.50 is the cost of 2kg of nickel. Actually, it can be gotten cheaper in these quantities, but this is an outside number.
| |
|
| |
|
| We need to look into purchasing in bulk as it is far cheaper than this sheet. Those guys must be making generous profit to say the least.
| | |From= |
| | *{{3D Printer}} - Casing |
| | *[[Controller Box]] - Power |
| | *{{Rod and Wire Mill}} - Wires, Tubes |
|
| |
|
| Wikipedia indicates that the price of metallic nickel these days is about 13 USD a kilogram, but it has been in the fifties during certain price spikes, I could not find a historical record, or even the current commodity price anywhere.
| | |Creates= |
| | *[[Electricity]] |
|
| |
|
| There are several ways to make the nickel electrode and active material which require different raw materials. See electrode sections for details. To both produce the nickel oxyhydroxide or hydroxide either inside the electrode matrix or as a powder of good particle size and porosity, which is then mixed into a paste and pasted into (with a matrix electrode) or onto (with a metal plate electrode, needs other additives to the paste) the electrode, there are several established economical ways that involve different raw materials, all of which will have different prices and availabilities:
| | |Uses= |
|
| |
|
| -Use a metallic nickel which is then oxidized electrochemically in a suitable chemical bath
| |
| - Use the oxyhydroxide or hydroxide powder, which can be purchased directly in purities adequate for battery use
| |
| - Nickel oxide (NiO2 I think), which is then roasted in air to oxidize it to the oxyhydroxide
| |
| - Nickel nitrate, sulfate, and potentially other salts can be melted, the electrode dipped in, and then the electrode dipped in sodium hydroxide to convert the nickel through a binary reaction to nickel hydroxide
| |
|
| |
|
| As long as we have nickel compound of adequate purity it's just a matter of figuring out an economical production method, or using an existing one. Which material will be needed can be chosen on price, availability and the ease and economy of the associated manufacturing technique.
| | |Enables= |
| | *{{Universal Power Supply}} - Stores energy |
| | *[[Charge Controller]] |
| | *[[Inverter]] |
|
| |
|
| Nickel could be recovered from the waste stream too, but this might not end up saving any money.
| | |Components= |
|
| |
|
| Iron as an element or in the form of steel is cheap, but a look on alibaba.com indicates that it may cost substantially more in pure iron powder. We might want to make our own in production, especially if a design which only uses a fraction of the iron as active material is chosen. The first problem is purification, in which the carbonyl process or other industrial processes might be useful in scaled down form, but this is where the contributions of a chemist would be particularly welcome as there is probably something which is more practical. Then it needs to be powdered either mechanically or through chemical or electrochemical processes, or some combination thereof.
| | }} |
|
| |
|
| The Edison process for producing the iron powder was much like what references indicate is the current process used for producing the powder for pocket plate batteries [the battery handbook, 3rd edition]:
| | =Components= |
| | ==Anode Compound== |
|
| |
|
| "pure iron" (how pure, what impurities are problematic can be nailed down to a fair degree from the documents listed in the sticking points section) is dissolved in sulfuric
| | * iron plate - low carbon, mild steel (demo) |
| acid.
| | * iron graphite compounded (Edison) |
| The FeSO4 is then re-crystallized, dried, and roasted (815 to 915�C) (in what atmosphere?) to Fe2O3. The material is washed free of any remaining [iron] sulfate, dried, and partially reduced in hydrogen.
| | * iron oxide |
| The resulting material (Fe3O4 and Fe) is partially oxidized, dried, ground, and blended. Small amounts of additives, are blended in to increase battery life, depassivate the iron electrode (remove the outer layer of iron dioxide), reduce gas production (what?), and improving conductivity. See side reaction and additives sections for details.
| | **http://en.wikipedia.org/wiki/Iron(II,III)_oxide |
| Note that there is some iron oxide in there too, that is an active material too and is used in the battery.
| |
|
| |
|
| ==Price of Nickel and Iron== | | ==node Construction== |
| http://www.indexmundi.com/commodities/?commodity=nickel price is very roughly $23 per kg. Pretty good really.
| |
| http://www.steelonthenet.com/commodity_prices.html price $0.60 per kg for scrap steel, presumably pure iron would be in that range.
| |
|
| |
|
| | * plain plate (demo) |
| | * pocket plate with mesh inserts (Edison) |
|
| |
|
| From the electrochemistry figures above for a 1 kWh unit, that would be $48 or so on metals. So 20 Wh per $ ($0.05 per Wh). Even the cheapest lead acid batteries are 7 Wh per $. In reality they are more like 4. So the good news it that materials cost should not sink the ship anyway, although I'm sure all the other costs for the perforated pocket, assembly etc. will add up plenty fast.
| | ==Cathode Compounds== |
|
| |
|
| ==Toxicity== | | * [http://en.wikipedia.org/wiki/Nickel%28III%29_oxide-hydroxide Nickel(III) oxide-hydroxide] |
| Nickel itself that is the concern rather than any particular compound. Water soluble compounds are unsurprisingly much more of a concern than metal that is bound in solid objects like nickel plating or some types of stainless steel, since it is more bioavailable and can be spread and spilled more easily. At low levels of exposure talking in terms of nickel content is done. But of course the exact solubility and other factors has a substantial effect on the exact toxicity at higher levels of different water soluble compounds too, note the ld50 is 10x lower for nickel chloride as for nickel oxyhydroxide according to the documents below, so the expedient of speaking in terms of nickel content doesn't always work that well.
| | :* [https://www.spectrumchemical.com/OA_HTML/chemical-products_NickelII-Chloride-Anhydrous-for-General-Organic-Chemistry_TCI-N0850.jsp?sitex=10020:22372:US§ion=25807 Nickel (II) chloride] |
| | :* [http://en.m.wikipedia.org/wiki/Sodium_hypochlorite#section_3 bleach] |
| | * nickel hydrate and pure nickel flake (Edison) |
|
| |
|
| Note that nickel hydroxide, oxide and oxyhydroxide are all considered "insoluble" although obviously the chemistry here depends on them being at least a bit soluble. But that increases the safety margin a bit. It may be substantially higher under these alkali conditions though.
| | http://en.wikipedia.org/wiki/Nickel%28II%29_hydroxide |
|
| |
|
| Still all metals are toxic to some degree including iron and we need some numbers to make an informed decision. Preferably a solid evaluation of dose response relationship, and if the batteries are to be widely adopted now is the time to factor in chronic exposure too. It needs to be considered not in a vacuum but relative to other options available.
| | Nickel(II) carbonate [http://en.wikipedia.org/wiki/Nickel%28II%29_carbonate can be combined with water to form Nickel(II) oxide], which can be used in cells. It generates Carbon Dioxide when mixed with water, which may affect Potassium Hydroxide in solution. Mix with water and allow to complete outgassing and dry before building the cell. |
|
| |
|
| higher quality :
| | Nickel(III) hydroxide "Nickel Oxide Black" and Nickel(II) carbonate "Green" are used as clay/ceramic colorants and available cheaply from ceramic and pottery related websites ... and though the purity is considered lower, it is still usuable for experimentation at a much lower cost. |
| http://jas.fass.org/cgi/content/abstract/28/5/620
| |
| http://www.annclinlabsci.org/cgi/content/abstract/11/2/119
| |
| http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1236757324101 l
| |
| http://www.epa.gov/iris/subst/0271.htm
| |
| https://fscimage.fishersci.com/msds/53189.htm
| |
| http://www.nickelinstitute.org/index.cfm/ci_id/13029/la_id/safe_use_guide_5.cfm.htm
| |
|
| |
|
| unknown due to lack of access:
| | ==Cathode Construction== |
| http://www.ncbi.nlm.nih.gov/pubmed/19888907
| |
| http://www.annclinlabsci.org/cgi/content/abstract/7/5/377
| |
| Low quality:
| |
| http://www.crios.be/Nickel/toxicology.htm
| |
| material safety datasheets with information regarding nickel oxyhydroxide, clearly it is sometimes used as a material in NiMH, in fact most of the hits are datasheets for such batteries rather than the material itself:
| |
| http://www.rdbatteries.net/Data/Panasonic_NiMH_Info.pdf
| |
| http://www.chiefsupply.com/resources/msds/Moto-NiCd.pdf
| |
| http://www.batteriesplus.com/msds/Duracell_Nickel_Oxyhydroxide_%20Batteries_NorthAmericaMSDS.pdf (as if companies would give accurate information on their own products)
| |
| http://www.it.pg.com/productsafety/msds/fabric_and_homecare/duracell/Duracell_Nickel_Oxyhydroxide_Batteries_(North_America_MSDS).pdf
| |
|
| |
|
| Note that the permissible exposure limits (PEL) for the material is about a fifth that of graphite, which we know is not too toxic though it might accumulate in the lungs I guess. Still there are a lot of variables there as most particles inhaled are not retained, depends on size etc etc.
| | * plain plate (demo) |
| | * pocket plate with mesh inserts (Edison) |
| | * generally nickel-plated rather than pure nickel |
|
| |
|
| Obviously the LD50 is quite low at 1000 mg/kg range. But that means little in terms of what happens at lower levels. For Chloride it seems to be in the 100 mg/kg range.
| | * Nickel sponge |
| | * Sintered nickel powder |
| | * Nickel mesh/cloth |
|
| |
|
| | ==Electrolyte== |
|
| |
|
| http://www.osha.gov/SLTC/healthguidelines/nickelsolublecompounds/recognition.html They fail to say dosages in critical places, this information is actually low quality.
| | * aqueous potassium hydroxide |
| | :* [https://www.spectrumchemical.com/OA_HTML/chemical-products_Potassium-Hydroxide-Pellets-Reagent-ACS_P1315.jsp?sitex=10020:22372:US§ion=15947 link] |
| | :* Water (121g per 100ml) |
| | * sodium hydroxide (alternate, lower voltage) |
| | * lithium (modern additive) |
|
| |
|
| nickel chloride:
| | ==Cell Casing== |
| http://www.sciencelab.com/xMSDS-Nickel_chloride-9926213
| |
|
| |
|
| oxide:http://www.inchem.org/documents/ukpids/ukpids/ukpid70.htm
| | * nickel-plated steel box, rubber seals (Edison) |
| | * plastic box (modern commercial) |
| | * glass jars (demo projects) |
| | * pvc cylinders (Ed's Workshop) |
|
| |
|
| From Dietary information perspective:
| | =Status= |
| http://iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/ULs%20for%20Vitamins%20and%20Elements.pdf
| | The '''Nickel-Iron Battery''' is currently in the [[Nickel-Iron_Battery/Research_Development|research phase of development]]. |
|
| |
|
| "A Tolerable Upper Intake Level (UL) is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. Unless otherwise
| | =See Also= |
| specified, the UL represents total intake from food, water, and supplements."
| | * [http://en.wikipedia.org/wiki/Battery_(electricity) Wikipedia: Battery] |
| | * [http://en.wikipedia.org/wiki/Nickel_iron_battery Wikipedia: Nickel Iron Battery] |
| | * [http://www.beutilityfree.com/content/index.php?option=com_content&view=article&id=44&Itemid=129 Battery Lifetimes] |
|
| |
|
| 1 milligram per day for nickel, 40 for iron. Well that's to totally zero adverse effect level and there's probably plenty of room for more there. Nickel is probably available as a dietary supplement, I wonder what happens when people take too much.
| | ==Other Communities== |
|
| |
|
| http://www.eoearth.org/article/Public_Health_Statement_for_Nickel case report little more than half way down of people drinking 250 mg/liter (250 ppm) nickel | | http://offgrid2.altervista.org/viewforum.php?f=7 |
| welding, relevant to pocket production:
| |
| http://www.crios.be/Welding/toxicology.htm Maybe it woudl be better to make the pockets without welding. Folding perforated sheets cleverly, sewing with nickel wire etc. plenty of other options that should be fine .
| |
|
| |
|
| As usual it is difficult to obtain quality information on a health issue and it is very time consuming to wade through the crap and get some answers. Case reports, vague qualitative statements, contradictions, and paywalls, are the norm. Ideally a table of dose-response relationships for a range of different people over a wide range of doses would be obtained but whatever.
| | =Off-the-Shelf= |
|
| |
|
| In summary it looks like it is certainly less problematic than lead or mercury, gloves, ladling or scooping rather than pouring dry materials, and working outside would more than suffice for me personally. Spills of solutions and especially powders should be prevented.
| | There's a number of suppliers of Ni-Fe batteries today. |
|
| |
|
| ==Sticking points and contributions imminently needed== | | ==Sichuan Changhong Battery Co., Ltd== |
|
| |
|
| We need to obtain the documents with the titles below. They are almost all available through sciencedirect.com, just search the titles. Linking to them directly is not possible because of the way they do the URLs. ''' If you can obtain these in some way, please do so without delay.''' I suggest temporarily changing your browser's default download folder to a new folder to fill up or something for efficiency.
| | It looks like most US companies selling Ni-Fe were white-label resellers of imported batteries manufactured by Sichuan Changhong Battery Co., Ltd (SCBC) in Mianyang, China. This is a huge manufacturer; I don't think you can buy direct from them. |
|
| |
|
| Then these can be legally shared by e.g. zippyshare.com with other developers who ask for a copy under the fair use doctrine.
| | # [https://qualmega.com/scbc.html Qualmega, Inc.] is "the exclusive distributor of Sichuan Changhong Battery Co., Ltd (SCBC)". They have an [[:File:Qualmega_Nickel_Iron_Battery.pdf|excellent brochure]] that describes the manufacture of the batteries in great detail. For example, they describe the construction method for the electrodes and state the battery case is made from MBS or PP (exact dimensions given for each Ah). |
| | # Iron Edison was one of the most popular resellers of Changhong batteries<ref>https://www.terravolt.net/iron-edison</ref>, but they went bankrupt during COVID (June 2023) |
| | # Zapp Works in Montana, USA (defunct) |
| | # [https://beutilityfree.com/ Be Utility Free] has been selling Ni-Fe batteries longer than Iron Edison |
|
| |
|
| We basically want all papers that mention nickel iron specifically and most of the others that relate to battery electrodes made from nickel oxyhydroxide(very frequently referred to as only "hydroxide" in the context of NiMH especially), and metallic iron and/or iron oxides. The electrode ones may not mention "nickel iron" per se because e.g. a good iron electrode can also be used in several other battery chemistries.
| | ==Seawill Technology Co., Ltd.== |
|
| |
|
| -To produce a battery that is economical, relatively easy to make, and which works to satisfaction (~0.2C, equal to or greater than 60% round trip efficiency) with efficient use of prototyping time, we need this information. I volunteer to read them all and come up with a plan for the next prototype(s) but do not have access. I can go back and get the full citations if they are for some reason needed. -Gregor
| | Another company (listed as both a manufacturer and a trader on alibaba) that sells Ni-Fe batteries in China is [https://seawill.en.alibaba.com/ SeaWill]. You *can* buy direct from them. |
|
| |
|
| ''Most important:'' | | ==Cost== |
| | *$4k for a 4.3kWhr usable capacity battery - see [https://beyondoilsolar.com/product/nickel-iron-battery-industrial-series/]. |
| | *Note that if this is authentically 4.3kW and it lasts 30years - then this is 1/2 the cost of a 24kWhr $4k forklift battery. A forklift battery will last 5 years [https://www.google.com/search?sxsrf=ALeKk026QG9BbsZl0rNsBS9KmerMgczY_w%3A1602863734253&source=hp&ei=dsKJX6q8DJDYsAXHkpG4Cw&q=how+long+does+a+forklift+battery+last&btnK=Google+Search&oq=how+to+configure+nvidia+geforce+gtx+1650+super+on+linux+mint&gs_lcp=CgZwc3ktYWIQAzIFCCEQoAEyBQghEKsCMgUIIRCrAjoOCAAQ6gIQtAIQmgEQ5QI6DgguELEDEMcBEKMCEJMCOggILhDHARCvAToLCC4QsQMQxwEQowI6CAgAELEDEIMBOggILhCxAxCDAToFCAAQsQM6AggAOgQIABAKOgYIABAWEB46CAghEBYQHRAeOgcIIRAKEKABUMsQWOmzAWD8tAFoBnAAeACAAeQGiAGhS5IBDTExLjQ3LjMuMi42LTGYAQCgAQGqAQdnd3Mtd2l6sAEG&sclient=psy-ab&ved=0ahUKEwiqz4DcvLnsAhUQLKwKHUdJBLcQ4dUDCAk&uact=5] - so this is 1/6 the length of the NiFe lifetime - or equivalent 4kW over the same time period. However, the beyondoilsolar.com link above says that electrolyte replacement at 30 years gets you another 30 years. If that is true, then we have 60 years life - and 1/2 the cost of lead acid batteries. |
| | *Disadvantage is slow discharge at C/2 rate max. For a 100A bat at 48V, that is 2400W. Plenty. |
| | *'''Summary - the up-front cost is steep - but lifetime considerations make this a very attractive offer.''' |
| | *Once open sourced, cost should go down 2x-5x still. |
| | *If we use Lead Acid for long life - 10% DoD - 2.4kW - for 20 year life - that is 3x more expensive than Nickel-Iron batteries over a 30 year life, and 6x more expensive over a 60 year life. |
|
| |
|
| Assessment of performance characteristics of the nickel---iron cell
| | =Links= |
| | *Good technical description on construction, including patents - '''[[Edison Battery]]''' |
| | *Energy density is 13 Whr/lb. Compare to Li-Ion at 10x this. |
| | *Paper on reconditioning 85 year old batteries - [http://www.nickel-iron-battery.com/Edison%20Cell%20Rejuvenation%2085%20yr-old%2013.%20DeMar.pdf] |
| | *'''Critique of Nickel Iron batteries - [https://forum.solar-electric.com/discussion/14736/compare-nickel-iron-edison-batteries-and-chinese-ni-fe-cells]''' - says that deep discharge destroys them. |
| | *Considerations for NiFe as starting batteries - [[Nickel-Iron SLI Battery]] |
| | *[[Nickel-Iron Battery/Prototype]] |
| | *Emails and communications - [[Battery Collaboration]] |
| | *See also Category:Nickel-Iron Battery Prototypes |
| | *[[Nickel-Iron Battery/Chemistry]] |
| | *Detailed construction of the battery is described in the book at [[Nickel-Iron_Battery/Manufacturing_Instructions]] - this is the best study of industry standards and taking off point for development. P. 14 in the PDF shows construction details of the battery. |
|
| |
|
| | | {{GVCS Footer}} |
| SECONDARY BATTERIES - NICKEL SYSTEMS Nickel–Iron
| |
| | |
| SECONDARY BATTERIES - NICKEL SYSTEMS Electrodes: Nickel
| |
| | |
| SECONDARY BATTERIES - NICKEL SYSTEMS
| |
| | |
| SECONDARY BATTERIES - NICKEL SYSTEMS Electrodes: Iron
| |
|
| |
| The nickel/iron battery
| |
| | |
| A nickel-iron battery with roll-compacted iron electrodes
| |
| | |
| Developmental studies on porous iron electrodes for the nickel---iron cell
| |
| | |
| The electrochemical generation of ferrate at pressed iron powder electrode: comparison with a foil electrode
| |
| | |
| 6V, 60Ah nickel-iron battery.
| |
| [http://md1.csa.com/partners/viewrecord.php?requester=gs&collection=TRD&recid=2274533EA]
| |
| Bulletin of Electrochemistry. Vol. 6, no. 2, pp. 263-265. 1990 <--- not available through sciencedirect.com
| |
| | |
| ''Less important but still highly desirable:''
| |
| The role of FeS and (NH4)2CO3 additives on the pressed type Fe electrode
| |
| | |
| Passivation of iron in alkaline carbonate solutions
| |
| | |
| Electrochemical characteristics of iron carbide as an active material in alkaline batteries
| |
| | |
| Temperature limitations of primary and secondary alkaline battery electrodes
| |
| | |
| 97/03847 Performance characterization of sintered iron electrodes in nickel/iron alkaline batteries
| |
| | |
| | |
| | |
| Nickel-based rechargeable batteries
| |
| | |
| | |
| Performance characterization of sintered iron electrodes in nickel/iron alkaline batteries
| |
| | |
| On the key importance of homogeneity in the electrochemical performance of industrial positive
| |
| | |
| active materials in nickel batteries
| |
| | |
| Electrochemical behaviour of Teflon-bonded iron oxide electrodes in alkaline solutions
| |
|
| |
| Rechargeable alkaline iron electrodes
| |
| | |
| Performance characterization of sintered iron electrodes in nickel/iron alkaline batteries
| |
| | |
| Role of activation on the performance of the iron negative electrode in nickel/iron cells
| |
| | |
| Rechargeable alkaline iron electrodes
| |
| | |
| Iron/carbon-black composite nanoparticles as an iron electrode material in a paste type
| |
| rechargeable alkaline battery
| |
| | |
| Research, development and demonstration of a nickel—iron battery for electric vehicle propulsion there are several papers with this term
| |
| | |
| The role of FeS and (NH4)2CO3 additives on the pressed type Fe electrode
| |
| | |
| There are some less important ones on the research page.
| |
| | |
| ==Future Prototypes==
| |
| It looks as though using a high surface area electrode made from nickel or another conductive material which is acceptable from a chemistry standpoint, then causing the oxyhydroxide to deposit on it electrochemically could be done. But there are a great many ways to make the electrodes, and some may be more suitable. A great number of patents are available with what look like better options. See the research page for links.
| |
| | |
| Note that in electrochemistry the cathode is the electrode to which cations are attracted. In other words the positive electrode, when when talking about the exterior of the battery could be called the anode. This is due to historical reasons.
| |
| | |
| All of them include some "plaque" or conductive matrix with fairly high surface area which extends throughout the active material. Nickel sponge, sintered nickel powder, and nickel fabric cloth like material, have been used.
| |
| | |
| A typical fibrous mesh for a battery that performs well at 0.2 C might use fibers 15 microns in width, usually not circular but their cross section might be 15 x 30 microns, which fill 20-10% of the volume of the electrode, which entails a void width of 60 microns ( (1000*(1-f)/((f*1000)/w) where f is the fill fraction, and w is the average width of the average fiber). Fibers as small as 2 microns have been used and they provide superior performance that might be good for a starting battery.
| |
| | |
| Other documents indicate that around 10-100 micron fibers with voids 100 to 200 microns apart in which the conductive material consumes 10 to 30 percent of the volume of the electrode is reasonable, so that's about the right range, the documents are talking about different contexts and from different eras so it's no surprise there is that variation.
| |
| | |
| A polymer binder loaded with graphite or other carbon particles which is then carbonized (not the same as pyrolized, entails changes in crystal structure and interconnection of the carbon mass which increases strength and conductivity) can be made, with the carbonized polymer being the conductive grid. Polymer binders (not carbonized) filled with conductive particles and hydrophobic plastic (e.g. teflon) spacer particles are often used in modern batteries. The mix is made, then pressed with great force onto a nickle or nickel plated grid or cloth. The polymer mix material can also be applied to a thin textured sheet of metal.
| |
| | |
| Fine flakes ("flitts") or fibers of nickel metal can be mixed with active material, pressed into a plate, and heated to melt or diffusion bond the flakes/fibers together, producing a conductive matrix surrounded and filled by a porous mass of oxyhydroxide.
| |
| | |
| Nickel wool or other low density fibrous masses of conductive material which are chemically acceptable might do as well. It is stated in patents that nickel plated steel wool works well. In this case the active material has to be applied in a paste or though more complex means like electrodeposition, in which the active material is precipitated by electrolysis in the electrode volume.
| |
| | |
| The so called pocket electrode is still used in modern batteries; conductive powder or fibers or flakes (graphite, nickel or nickel cobalt alloy flitts) a couple millimeters in size are mixed with the active material and pressed into "pockets" formed from perforated metal sheets. The pressing is needed to get good electrical contact between the particles and conductive material.
| |
| | |
| For the iron electrode high surface area solid iron electrode may be used plain or with small amounts of something to provide sulfide ions like magnesium sulfide, iron sulfide or even elemental sulfur, to cause activation of the electrode (removal of the iron monoxide layer as the sulfur is more electronegative than the iron). For high purity powder like carbonyl iron the sulfide needs to be added. Otherwise it is often present in high enough amounts for some batteries as an impurity (see other reactions section). The appropriate surface area of both electrodes which will give a reasonably low internal resistance needs to be calculated or tested. References indicate it should be on the order of 10 sq meters per gram, which is about 26 micron average particle diameter (density of NiOOH is 4 so 40 square meters or 400,000 sq cm per cc, so pi*r^2*4/(4*pi*r^3/3)=1/(r/3)=400,000 so r=1/133,333 cm or 13.3 microns, so diameter is 26.6 microns. This same reference source, the battery handbook 3rd edition, says that 200 mesh (0.075 or 75 micron diameter) particles are used for pocket plate technology so there is some disconnect there.
| |
| | |
| Loose powder is not ever used, because the conductivity between particles is too low. In the the case of the nickel electrode, they must be at least pressed together (pocket type) or surrounded with a fine conductive matrix and have quite small size area and then during the initial charge/discharge cycles they tend to bond together so the bulk conductivity of the mass increases to an acceptable level (pasted). This is because the nickel oxyhydroxide is not very conductive (see other reactions section). Nickel hydroxide is even less conductive (how much?). This may help to explain why the electrodes are rarely loaded with the hydroxide initially, as the lower conductivity combined with the poor level of inter-particle contact before the particles are bonded together would make the initial charge/discharge cycles take that much longer.
| |
| | |
| | |
| In at least one of the Edison batteries he chose to use mercury to increase the conductivity between iron particles but that may have been a battery intended for Starting and lighting (SLI) in cars, which requires a very low internal resistance as discharge rates can exceed 20C during starting. We need no more than 1C or at most 2C (lithium ion are 2C or so usually) for general use and short term load leveling, and 0.1 may do (just) for solar power system energy storage. If we did need high currents sintered electrodes would be more sensible. Commercial batteries like the Changhong batteries intended for solar use are rated for 0.2 C but they make batteries capable of 10C for starting locomotives too. The C ratings are only guidelines however and can be exceeded greatly at cost of efficiency and effective battery capacity - 6 C produces a capacity of 65% rated capacity for the battery in the sealed battery testing doc.
| |
| | |
| Over time the electrode shape could change in undesirable ways, reducing surface area and increasing the battery's internal resistance to an excessive value. It appears that this occurs to a relatively small degree in nickel iron batteries, and in fact this is mainly what gives them their much longer life compared with other batteries. Mostly it is limited by the very low solubility of the reactants and reaction products, they cannot travel far in the electrolyte before being redeposited (precipitating out of solution). In fact the nickel electrode reactions are thought to occur almost all in the solid state.
| |
| | |
| This is one of the reasons deep discharging of lead acid is a problem. Although there are many different types of lead acid battery there is usually alloy of antimony and lead used to form the electrode scaffold for one or both electrodes, which reacts more slowly than the lead that is supposed to cover it. But if discharged too deeply the scaffold will react too, and it cannot be reformed in the shape it was, rendering the battery damaged. Similarly during recharge some battery types form dendrites from one electrode to another - thin shafts of metal. As the finger of metal protrudes towards the opposing electrode the resistance between the tip of the finger and the opposing electrode gets lower, resulting in a higher current at the tip of the dendrite, causing metal to be preferentially deposited at the tip of it, lengthening it until it touches the opposite electrode, shorting the battery. Clearly over many charge/discharge cycles the cumulative effects that result from the relative effect size of these processes can cause substantial changes in electrode shape if they are not understood and accounted for.
| |
| | |
| In the nickel iron battery the fact that this occurs very little allows the iron electrode metal scaffold (plaque, current collector) to be made of the reactant itsself. The surface can get converted to the reaction product and back again many times without changing the shape of the internal iron structure. Usually, if you did this with e.g. a zinc anode the zinc would get out of shape pretty fast due to dendrite growth etc. and become useless. A sintered block with 3 to 4 times the amount of iron than stoichiometric is typical, so 1/3 to 1/4 of the iron is used in each full charge/discharge.
| |
| | |
| In patents it appears to be universally assumed that for both electrodes, if they have a high surface area at manufacture they continue to have a high surface area thereafter so this may not be a problem.
| |
| | |
| =Other important reactions in the cell=
| |
| | |
| If you want a good battery rather than a poor one, the side reactions and other reactions in the cell are the most critical factor, with the only second being electrode geometry/design. They are a fundamental part of battery design. These exclude the reactions that contribute to the output electrical energy to the cell.
| |
| | |
| These are what sap energy away, leading to poor efficiency and high self discharge, and partly what limits battery life. Some of them are used to counteract the undesirable ones.
| |
| | |
| There are others that need to be learned from the documents listed in the sticking points section.
| |
| | |
| -self discharge due to oxidization by dissolved o2 (pretty small in magnitude)
| |
| | |
| -and the iron being anaerobically attacked by water, about 1000 times larger in magnitude than the oxidation due to dissolved o2. This is the main reason the battery has such a high self discharge rate.
| |
| | |
| - self discharge at the nickel electrode need to check the mechanisms/reactions again probably relatively low since it is in nimh and nicad despite the same reactants present.
| |
| | |
| -corrosion of the metallic mes h by electrolyte, this may be one of the lifetime-limiting reactions that increases with temperature.
| |
| | |
| -oxidization of sulfide to sulfate and it's ensuing accumulation on the surface of the iron electrode, increasing internal resistance. Sulfate is also needed to keep the iron electrode active so when it is used up the battery dies.
| |
| | |
| -accumulation of sulfur on the surface of the iron electrode esp. at lower temperatures and high discharge rates.
| |
| | |
| -deposition of iron in the nickel compound crystal structure <--needs more research
| |
| | |
| -oxidization of the surface of metallic flitts to low conductivity nickel oxide.
| |
| edison had a problem with nonconductive layer on the flitts forming didn't know what it was maybe explained in later patents.
| |
| | |
| -carbonate and the other one in the battery hadnbook undesirable ions <-- need to add those other ones.
| |
| | |
| | |
| | |
| probably all kinds of minor undesirable contaminants, edison mentiones manganese , high purity iron like carbonyl iron is often used same for nickel electrode. Howevetr this may be expensive
| |
| | |
| lithium hydroxide apparently improves the thermodynamic reversibility of reactions and slows down the iron poisoning of the nickel elecrode maybe this indicates longer life and higher charge/discharge efficiency , we need a chemisty who can identify other ways of improving esp the charg/discharge efficiency by identifying other acceptable additives that might work.
| |
| | |
| -evolution of gasses at the nickel electrodes during charging, this could play into the ow efficiency but may only be commesurate with other prerequisite reactions at the iron electrode and unpreventable in themselves. Another contributor to low charge/discharge efficiency maybe.
| |
| | |
| - Evolution of gasses at electrode during periods when a charged battery sit there for future use (charge-stand), again in conjunction with prerequisite activity at the iron electrode. Another cause of self discharge.
| |
| | |
| -The air interface one that causes oxidization of the nickel metal and/or active material(?) of the electrode when there is an interface with the electrolyte and air, nickel electrode needs to be submerged. Another cause of self discharge if not designed right, plus could corrode the nickel possibly leading to failure.
| |
| | |
| Need to add references but should have the sci docs first or will take forever and be low quality refs anyway.
| |
| | |
| =Additives=
| |
| | |
| - cobalt hydroxide is used to improve conductivity of the nickel electrode either by adding it to the nickel oxyhydroxide at manufacture or into the powder after, by e.g. coating the particle electrolytically to give them a higher effective conductivity without interfering with their reactivity too much (it does reduce it a little though so again there will be an optimum amount). It can also help to increase the fraction of active material that is utilized. 1 to 5% of the active material mass may be used for this. We probably want to avoid bothering with this.
| |
| | |
| - cobalt to the metallic mesh, function needs to be checked, probably to improve contact resistance and reduce oxidization of the mesh and maybe reduce corrosion rate by electrolyte.
| |
| | |
| | |
| =Other factors affecting performance=
| |
| At low temperatures with some iron electrode designs sulfur can accumulate on the iron electrode and increase internal resistance (designs which incorporate elemental sulfur, patents indicate this can be rectified by using a sulfide salt of low solubility like iron sulfide FeS).
| |
| | |
| The conductivity of the electrolyte goes down as the temp goes up. This is desirable but other undesirable reactions increase in rate at higher temps so there is a compromise there. Also this is one of the main mechanisms that can lead to thermal runaway during constant voltage charging as it can form a positive feedback loop. The solution is to just not use constant voltage charging, which is easy.
| |
| | |
| The fraction of the battery that is actually active material, and the fraction of material that is available for actual use all vary quite substantially with the design of the electrodes, and the amount of electrolyte added etc.
| |
| | |
| The factors like the energy to weight ratio and power to weight will of course tend to be affected by any non-reactant materials used to e.g. reduce cost etc, but fortunately those are of little importance in the context of OSE. Basically we want to produce something that can replace lead acid which is cheaper, easier to work with and make and maintain, more durable with abuse and longer lasting, and not as bad for the environment. Ideally both for starting lighting ignition (SLI) batteries and also storage. Batteries described in documents can handle 6C and more without seriously hard to manufacture materials and additives so SLI is definitely an option.
| |
| | |
| =factors affecting cost=
| |
| Not all of the active material actually gets used in the electrode. Obviously we want it to be high in the nickel electrode in particular because the cost of the material is high.
| |
| | |
| In the Edison cell the fraction of mass utilized can be calculated from the weights and composition of the electrodes Edison gives in the patents and this should be done. It can be increased with the addition of conductive carbon (like graphite) particles to the active material. Documents indicate that the homogeneity of the particle sizes is important in pocket cells to increase mass utilization, which should be no surprise as it would entail a smaller number of particles in between the gaps of large particles that are not compressed in any way against their neighbors, leading to high contact resistance and therefore low electrical coupling to the current collector.
| |
| | |
| For pasted electrodes it can be very high for both the iron and the nickel electrodes, patents indicate that it could be 80% without cobalt additive and almost 100% with it for the nickel. Smaller particle sizes and finer denser mesh helps too. Similar figures apply to the iron but since iron is cheap and nontoxic that will probably not be a deciding factor in the iron electrode design.
| |
| | |
| For sintered iron electrodes it is typically 1/3 or 1/4 of the total mass. The rest forms the conductive matrix of the current collector.
| |
| | |
| If the active material or a precursor that is later converted the the active material (like nickel oxide, probably NiO) is added before sintering the current collector that would need to be checked, but it would likely be high.
| |
| | |
| With electrodeposition and molten salt it should be very high.
| |
| | |
| For the nickel electrode the amount of conductive metallic material used (if metal is used, carbon works too) is typically 30% to 10 by volume of the electrode, so this will have a significant impact on cost if it is solid nickel. This may be a good reason to use a nickel plated steel wool current collector.
| |
| | |
| | |
| =Notes on interpreting patents and other documentation=
| |
| | |
| Chemists have a terrible habit of interchanging the names of compounds as if they were synonyms based on family relations, and glossing over details. Nickel Oxyhydroxide if often referred to as nickel hydroxide in this context. Incidentally so is actual nickel hydroxide, which can get confusing for someone with limited knowledge of the art.
| |
| | |
| A nickel-iron battery likewise may use not just iron as the negative electrode but can also make use of the higher iron oxides like Fe3O4 (ferric oxide) called iron oxide as a reactant (need to check details on this). Similarly FeO (ferrous oxide or iron monoxide) is a problem in the batteries but is likewise called iron oxide. Similarly there are many nickel oxides and the name is often used to refer to several.
| |
| | |
| There are also several different reactions that are important to contribute output energy in the battery, not just the one on the wikipedia page. (need to add details)
| |
| | |
| ==Related pages==
| |
| http://openfarmtech.org/wiki/Batteries
| |
| http://openfarmtech.org/wiki/Nickel-Iron_Battery/Research
| |

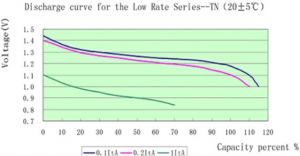 . Source - [4]
. Source - [4]