Nickel-Iron Battery: Difference between revisions
No edit summary |
|||
| (247 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{OrigLang}} | ||
{{GVCS Header}} | |||
=Overview= | |||
[[File:Nickel-Iron Batterypic.png|thumb|400px|Nickel Iron Battery]] | |||
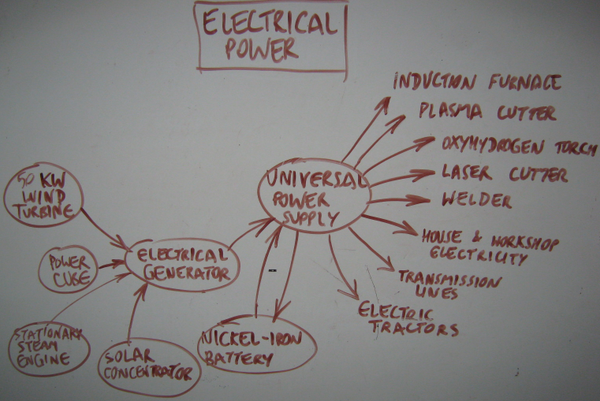
The '''Nickel Iron Battery is the only known lifetime design battery. These last 100 years, such as the Edison batteries unearthed after a century that work like new. Thus, it is the primary electrical energy storage device for the [[GVCS]], outside of indirect sources such as [[Compressed Air Energy Storage]], water [[Gravity Storage]], and storage of energy via [[Hydrogen Production]]. | |||
==Advantages== | |||
*'''Theoretically unlimited lifetime''': Long lifetime of 8-10 years - when you don't throw out the battery - just replace the electrolyte. [http://www.s4solar.co.nz/information/products/nickel_iron_battery/] | |||
*About X = $1000/kW - but unlimited life means that the full-cost accounted cost means that the cost is really X/N - where N is a number that you choose. Think of this - the core lives for ever - you replace the casing and electrolyte. | |||
*Open source design of electrodes | |||
*Cells can be made to any Ahr rating | |||
*Nickel and iron obtained from scrap stream, reprocessed via [[Induction Furnace]] | |||
*Completely closed loop material cycle ecology | |||
*Max discharge/charge = C/2. Optimal charge/discharge - C/4. [https://ironedison.com/images/products/Iron%20Edison/NiFe%20Industrial/Iron_Edison_Nickel_Iron_battery_spec_sheet_2017.pdf] | |||
*Vidoe showing build of a simple cell - [https://www.youtube.com/watch?v=K84PywMwjZg] | |||
*Discharge at 1C rate appears to get 70% of battery capacity? Not likely, it must be more like time - [[File:1cnickeliron.png|300px]]. Source - [https://www.alibaba.com/product-detail/Max-long-life-nife-nickel-iron_1986518612.html] | |||
= | ==Disadvantages== | ||
NiFe | *The historical NiFe technology’s notable limitations include low specific energy, | ||
low power, low charge retention and poor low temperature performance along with being high | |||
cost1 and is still in limited mass production throughout the world today for specific applications. | |||
NiFe battery chemistry is known for its robustness, extreme shelf and cycle life. The historical | |||
NiFe technology that was most robust to abuse also had limitations in being heavy, low power, | |||
low charge retention and poor low temperature performance along with being high cost. Thus, | |||
over the years, other nickel battery technologies (e.g., NiCd, NiMH, NiZn, and NiH2) have | |||
displaced NiFe in many applications. [https://www.osti.gov/biblio/1323594-selected-test-results-from-encell-technology-nickel-iron-battery]. This paper discusses the 'improved' Encell cell with much shorter lifetime. | |||
*OSE Assessment: | |||
**Low specific energy - still perfect for stationary applications of renewable energy | |||
**Low power - in a renewable energy scenario, large loads are run from PV when the sun is shining. Battery storage is only carry-over through the night for essential activity such as computers and internet - not heating or cooling (high-priority overnight cooling needs, such as refrigeration, can still be met by Ni-Fe using devices with an [https://en.wikipedia.org/wiki/Inverter_compressor inverter compressors]). Thus, low power is not an issue. | |||
**Low temperature performance - is an issue if batteries are outside in freezing temperatures. | |||
**High cost - that is not an accurate assessment. They have a higher up-front cost, but their lifetime cost is significantly lower. Lead acid survives because it is a good car starter battery, but for power storage, it does not do well. | |||
**C/2 max discharge rate. | |||
=The Latest= | |||
*2012 - '''Ultra Nickel Iron Bats sighted in Nature''' - high performance nanotech batteries have been shown to be 1000x more powerful than Edison's originals and nearly 100% [[Coulombic Efficiency]] - in 2012 - [https://www.nature.com/articles/ncomms1921]. Thus, the potential is there for a safe and durable battery - even in automotive applications. | |||
*2020 - '''Batolyzer - Combination battery and electrolyzer'''- has been demonstrated for producing hydrogen in addition to storing energy, thus killing 3 birds with one stone. Energy storage, hydrogen production, and [[SDG 7]]. See paper at [https://www.frontiersin.org/articles/10.3389/fenrg.2020.509052/full] | |||
*2017 - High discharge, sintereed iron electrodes, 2017 - '''3500 cycles at 100% depth of discharge'''. Potential for solving grid storage. [https://iopscience.iop.org/article/10.1149/2.1161702jes/pdf] | |||
= | =Detailed Description= | ||
The '''nickel-iron battery''' (NiFe battery) or "edison cell" is a storage battery having a nickel oxide-hydroxide cathode and an iron anode, with an electrolyte of potassium hydroxide (lye can be used as a substitute). | |||
The active materials are held in nickel-plated steel tubes or perforated pockets. | |||
It is a very robust battery which is tolerant of abuse, (overcharge, overdischarge, and short-circuiting) and can have very long life even if so treated. | |||
It is often used in backup situations where it can be continuously charged and can last for more than 20 years. | |||
'''Nickel-iron batteries''' have ~50 year lifetimes, compared to a few-year lifetime of lead acid batteries. They are environmentally more benign, and lend themselves to local recycling and fabrication. They can have higher discharge rates and faster charge times than lead-acid batteries depending on mechanical design of the electrodes etc, so they lend themselves not only to off-grid power, but also to power electronics applications such as welding and heavy workshop power. In China a company by the name of changhong batteries makes a version of them for use in automotive starter batteries. Their energy density is half that of lead-acid batteries, but their long lifetime and deep discharge ability makes them highly relevant to the [[GVCS]], including to electric farming equipment as the next generation of [[LifeTrac]] infrastructure. | |||
The [[Edison Battery]] was developed and promoted primarily by Thomas A Edison. | |||
=Product Ecology= | |||
[[Image:Electricalpowereco.png|600px|thumb|[[Product Ecology]]]] | |||
{{Product Ecology | |||
= | |Product={{Battery}} | ||
|From= | |||
*{{3D Printer}} - Casing | |||
*[[Controller Box]] - Power | |||
*{{Rod and Wire Mill}} - Wires, Tubes | |||
|Creates= | |||
*[[Electricity]] | |||
|Uses= | |||
|Enables= | |||
*{{Universal Power Supply}} - Stores energy | |||
*[[Charge Controller]] | |||
*[[Inverter]] | |||
|Components= | |||
}} | |||
=Components= | |||
==Anode Compound== | |||
* iron plate - low carbon, mild steel (demo) | |||
http:// | * iron graphite compounded (Edison) | ||
* iron oxide | |||
**http://en.wikipedia.org/wiki/Iron(II,III)_oxide | |||
==node Construction== | |||
* plain plate (demo) | |||
* pocket plate with mesh inserts (Edison) | |||
==Cathode Compounds== | |||
* [http://en.wikipedia.org/wiki/Nickel%28III%29_oxide-hydroxide Nickel(III) oxide-hydroxide] | |||
:* [https://www.spectrumchemical.com/OA_HTML/chemical-products_NickelII-Chloride-Anhydrous-for-General-Organic-Chemistry_TCI-N0850.jsp?sitex=10020:22372:US§ion=25807 Nickel (II) chloride] | |||
:* [http://en.m.wikipedia.org/wiki/Sodium_hypochlorite#section_3 bleach] | |||
* nickel hydrate and pure nickel flake (Edison) | |||
http:// | http://en.wikipedia.org/wiki/Nickel%28II%29_hydroxide | ||
Nickel(II) carbonate [http://en.wikipedia.org/wiki/Nickel%28II%29_carbonate can be combined with water to form Nickel(II) oxide], which can be used in cells. It generates Carbon Dioxide when mixed with water, which may affect Potassium Hydroxide in solution. Mix with water and allow to complete outgassing and dry before building the cell. | |||
Nickel(III) hydroxide "Nickel Oxide Black" and Nickel(II) carbonate "Green" are used as clay/ceramic colorants and available cheaply from ceramic and pottery related websites ... and though the purity is considered lower, it is still usuable for experimentation at a much lower cost. | |||
==Cathode Construction== | |||
* plain plate (demo) | |||
* pocket plate with mesh inserts (Edison) | |||
* generally nickel-plated rather than pure nickel | |||
http:// | * Nickel sponge | ||
* Sintered nickel powder | |||
* Nickel mesh/cloth | |||
==Electrolyte== | |||
* aqueous potassium hydroxide | |||
:* [https://www.spectrumchemical.com/OA_HTML/chemical-products_Potassium-Hydroxide-Pellets-Reagent-ACS_P1315.jsp?sitex=10020:22372:US§ion=15947 link] | |||
:* Water (121g per 100ml) | |||
* sodium hydroxide (alternate, lower voltage) | |||
* lithium (modern additive) | |||
==Cell Casing== | |||
* nickel-plated steel box, rubber seals (Edison) | |||
* plastic box (modern commercial) | |||
* glass jars (demo projects) | |||
* pvc cylinders (Ed's Workshop) | |||
=Status= | |||
The '''Nickel-Iron Battery''' is currently in the [[Nickel-Iron_Battery/Research_Development|research phase of development]]. | |||
=See Also= | |||
* [http://en.wikipedia.org/wiki/Battery_(electricity) Wikipedia: Battery] | |||
* [http://en.wikipedia.org/wiki/Nickel_iron_battery Wikipedia: Nickel Iron Battery] | |||
* [http://www.beutilityfree.com/content/index.php?option=com_content&view=article&id=44&Itemid=129 Battery Lifetimes] | |||
==Other Communities== | |||
http://offgrid2.altervista.org/viewforum.php?f=7 | |||
=Off-the-Shelf= | |||
There's a number of suppliers of Ni-Fe batteries today. | |||
==Sichuan Changhong Battery Co., Ltd== | |||
It looks like most US companies selling Ni-Fe were white-label resellers of imported batteries manufactured by Sichuan Changhong Battery Co., Ltd (SCBC) in Mianyang, China. This is a huge manufacturer; I don't think you can buy direct from them. | |||
# [https://qualmega.com/scbc.html Qualmega, Inc.] is "the exclusive distributor of Sichuan Changhong Battery Co., Ltd (SCBC)". They have an [[:File:Qualmega_Nickel_Iron_Battery.pdf|excellent brochure]] that describes the manufacture of the batteries in great detail. For example, they describe the construction method for the electrodes and state the battery case is made from MBS or PP (exact dimensions given for each Ah). | |||
# Iron Edison was one of the most popular resellers of Changhong batteries<ref>https://www.terravolt.net/iron-edison</ref>, but they went bankrupt during COVID (June 2023) | |||
# Zapp Works in Montana, USA (defunct) | |||
# [https://beutilityfree.com/ Be Utility Free] has been selling Ni-Fe batteries longer than Iron Edison | |||
==Seawill Technology Co., Ltd.== | |||
Another company (listed as both a manufacturer and a trader on alibaba) that sells Ni-Fe batteries in China is [https://seawill.en.alibaba.com/ SeaWill]. You *can* buy direct from them. | |||
==Cost== | |||
*$4k for a 4.3kWhr usable capacity battery - see [https://beyondoilsolar.com/product/nickel-iron-battery-industrial-series/]. | |||
*Note that if this is authentically 4.3kW and it lasts 30years - then this is 1/2 the cost of a 24kWhr $4k forklift battery. A forklift battery will last 5 years [https://www.google.com/search?sxsrf=ALeKk026QG9BbsZl0rNsBS9KmerMgczY_w%3A1602863734253&source=hp&ei=dsKJX6q8DJDYsAXHkpG4Cw&q=how+long+does+a+forklift+battery+last&btnK=Google+Search&oq=how+to+configure+nvidia+geforce+gtx+1650+super+on+linux+mint&gs_lcp=CgZwc3ktYWIQAzIFCCEQoAEyBQghEKsCMgUIIRCrAjoOCAAQ6gIQtAIQmgEQ5QI6DgguELEDEMcBEKMCEJMCOggILhDHARCvAToLCC4QsQMQxwEQowI6CAgAELEDEIMBOggILhCxAxCDAToFCAAQsQM6AggAOgQIABAKOgYIABAWEB46CAghEBYQHRAeOgcIIRAKEKABUMsQWOmzAWD8tAFoBnAAeACAAeQGiAGhS5IBDTExLjQ3LjMuMi42LTGYAQCgAQGqAQdnd3Mtd2l6sAEG&sclient=psy-ab&ved=0ahUKEwiqz4DcvLnsAhUQLKwKHUdJBLcQ4dUDCAk&uact=5] - so this is 1/6 the length of the NiFe lifetime - or equivalent 4kW over the same time period. However, the beyondoilsolar.com link above says that electrolyte replacement at 30 years gets you another 30 years. If that is true, then we have 60 years life - and 1/2 the cost of lead acid batteries. | |||
*Disadvantage is slow discharge at C/2 rate max. For a 100A bat at 48V, that is 2400W. Plenty. | |||
*'''Summary - the up-front cost is steep - but lifetime considerations make this a very attractive offer.''' | |||
*Once open sourced, cost should go down 2x-5x still. | |||
*If we use Lead Acid for long life - 10% DoD - 2.4kW - for 20 year life - that is 3x more expensive than Nickel-Iron batteries over a 30 year life, and 6x more expensive over a 60 year life. | |||
=Links= | |||
*Good technical description on construction, including patents - '''[[Edison Battery]]''' | |||
*Energy density is 13 Whr/lb. Compare to Li-Ion at 10x this. | |||
*Paper on reconditioning 85 year old batteries - [http://www.nickel-iron-battery.com/Edison%20Cell%20Rejuvenation%2085%20yr-old%2013.%20DeMar.pdf] | |||
*'''Critique of Nickel Iron batteries - [https://forum.solar-electric.com/discussion/14736/compare-nickel-iron-edison-batteries-and-chinese-ni-fe-cells]''' - says that deep discharge destroys them. | |||
*Considerations for NiFe as starting batteries - [[Nickel-Iron SLI Battery]] | |||
*[[Nickel-Iron Battery/Prototype]] | |||
*Emails and communications - [[Battery Collaboration]] | |||
*See also Category:Nickel-Iron Battery Prototypes | |||
*[[Nickel-Iron Battery/Chemistry]] | |||
*Detailed construction of the battery is described in the book at [[Nickel-Iron_Battery/Manufacturing_Instructions]] - this is the best study of industry standards and taking off point for development. P. 14 in the PDF shows construction details of the battery. | |||
{{GVCS Footer}} | |||
Latest revision as of 20:18, 18 May 2024
| Nickel-Iron Battery | ||
|---|---|---|
| Home | Research & Development | Bill of Materials | Manufacturing Instructions | User's Manual | User Reviews | 
| |
Overview
The Nickel Iron Battery is the only known lifetime design battery. These last 100 years, such as the Edison batteries unearthed after a century that work like new. Thus, it is the primary electrical energy storage device for the GVCS, outside of indirect sources such as Compressed Air Energy Storage, water Gravity Storage, and storage of energy via Hydrogen Production.
Advantages
- Theoretically unlimited lifetime: Long lifetime of 8-10 years - when you don't throw out the battery - just replace the electrolyte. [1]
- About X = $1000/kW - but unlimited life means that the full-cost accounted cost means that the cost is really X/N - where N is a number that you choose. Think of this - the core lives for ever - you replace the casing and electrolyte.
- Open source design of electrodes
- Cells can be made to any Ahr rating
- Nickel and iron obtained from scrap stream, reprocessed via Induction Furnace
- Completely closed loop material cycle ecology
- Max discharge/charge = C/2. Optimal charge/discharge - C/4. [2]
- Vidoe showing build of a simple cell - [3]
- Discharge at 1C rate appears to get 70% of battery capacity? Not likely, it must be more like time -
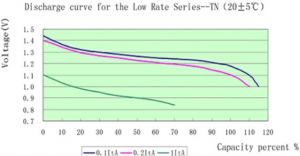 . Source - [4]
. Source - [4]
Disadvantages
- The historical NiFe technology’s notable limitations include low specific energy,
low power, low charge retention and poor low temperature performance along with being high cost1 and is still in limited mass production throughout the world today for specific applications. NiFe battery chemistry is known for its robustness, extreme shelf and cycle life. The historical NiFe technology that was most robust to abuse also had limitations in being heavy, low power, low charge retention and poor low temperature performance along with being high cost. Thus, over the years, other nickel battery technologies (e.g., NiCd, NiMH, NiZn, and NiH2) have displaced NiFe in many applications. [5]. This paper discusses the 'improved' Encell cell with much shorter lifetime.
- OSE Assessment:
- Low specific energy - still perfect for stationary applications of renewable energy
- Low power - in a renewable energy scenario, large loads are run from PV when the sun is shining. Battery storage is only carry-over through the night for essential activity such as computers and internet - not heating or cooling (high-priority overnight cooling needs, such as refrigeration, can still be met by Ni-Fe using devices with an inverter compressors). Thus, low power is not an issue.
- Low temperature performance - is an issue if batteries are outside in freezing temperatures.
- High cost - that is not an accurate assessment. They have a higher up-front cost, but their lifetime cost is significantly lower. Lead acid survives because it is a good car starter battery, but for power storage, it does not do well.
- C/2 max discharge rate.
The Latest
- 2012 - Ultra Nickel Iron Bats sighted in Nature - high performance nanotech batteries have been shown to be 1000x more powerful than Edison's originals and nearly 100% Coulombic Efficiency - in 2012 - [6]. Thus, the potential is there for a safe and durable battery - even in automotive applications.
- 2020 - Batolyzer - Combination battery and electrolyzer- has been demonstrated for producing hydrogen in addition to storing energy, thus killing 3 birds with one stone. Energy storage, hydrogen production, and SDG 7. See paper at [7]
- 2017 - High discharge, sintereed iron electrodes, 2017 - 3500 cycles at 100% depth of discharge. Potential for solving grid storage. [8]
Detailed Description
The nickel-iron battery (NiFe battery) or "edison cell" is a storage battery having a nickel oxide-hydroxide cathode and an iron anode, with an electrolyte of potassium hydroxide (lye can be used as a substitute).
The active materials are held in nickel-plated steel tubes or perforated pockets.
It is a very robust battery which is tolerant of abuse, (overcharge, overdischarge, and short-circuiting) and can have very long life even if so treated.
It is often used in backup situations where it can be continuously charged and can last for more than 20 years.
Nickel-iron batteries have ~50 year lifetimes, compared to a few-year lifetime of lead acid batteries. They are environmentally more benign, and lend themselves to local recycling and fabrication. They can have higher discharge rates and faster charge times than lead-acid batteries depending on mechanical design of the electrodes etc, so they lend themselves not only to off-grid power, but also to power electronics applications such as welding and heavy workshop power. In China a company by the name of changhong batteries makes a version of them for use in automotive starter batteries. Their energy density is half that of lead-acid batteries, but their long lifetime and deep discharge ability makes them highly relevant to the GVCS, including to electric farming equipment as the next generation of LifeTrac infrastructure.
The Edison Battery was developed and promoted primarily by Thomas A Edison.
Product Ecology
| From | Uses | Creates | Enables |
|---|---|---|---|
Components |
|
Components
Anode Compound
- iron plate - low carbon, mild steel (demo)
- iron graphite compounded (Edison)
- iron oxide
node Construction
- plain plate (demo)
- pocket plate with mesh inserts (Edison)
Cathode Compounds
- nickel hydrate and pure nickel flake (Edison)
http://en.wikipedia.org/wiki/Nickel%28II%29_hydroxide
Nickel(II) carbonate can be combined with water to form Nickel(II) oxide, which can be used in cells. It generates Carbon Dioxide when mixed with water, which may affect Potassium Hydroxide in solution. Mix with water and allow to complete outgassing and dry before building the cell.
Nickel(III) hydroxide "Nickel Oxide Black" and Nickel(II) carbonate "Green" are used as clay/ceramic colorants and available cheaply from ceramic and pottery related websites ... and though the purity is considered lower, it is still usuable for experimentation at a much lower cost.
Cathode Construction
- plain plate (demo)
- pocket plate with mesh inserts (Edison)
- generally nickel-plated rather than pure nickel
- Nickel sponge
- Sintered nickel powder
- Nickel mesh/cloth
Electrolyte
- aqueous potassium hydroxide
- link
- Water (121g per 100ml)
- sodium hydroxide (alternate, lower voltage)
- lithium (modern additive)
Cell Casing
- nickel-plated steel box, rubber seals (Edison)
- plastic box (modern commercial)
- glass jars (demo projects)
- pvc cylinders (Ed's Workshop)
Status
The Nickel-Iron Battery is currently in the research phase of development.
See Also
Other Communities
http://offgrid2.altervista.org/viewforum.php?f=7
Off-the-Shelf
There's a number of suppliers of Ni-Fe batteries today.
Sichuan Changhong Battery Co., Ltd
It looks like most US companies selling Ni-Fe were white-label resellers of imported batteries manufactured by Sichuan Changhong Battery Co., Ltd (SCBC) in Mianyang, China. This is a huge manufacturer; I don't think you can buy direct from them.
- Qualmega, Inc. is "the exclusive distributor of Sichuan Changhong Battery Co., Ltd (SCBC)". They have an excellent brochure that describes the manufacture of the batteries in great detail. For example, they describe the construction method for the electrodes and state the battery case is made from MBS or PP (exact dimensions given for each Ah).
- Iron Edison was one of the most popular resellers of Changhong batteries[1], but they went bankrupt during COVID (June 2023)
- Zapp Works in Montana, USA (defunct)
- Be Utility Free has been selling Ni-Fe batteries longer than Iron Edison
Seawill Technology Co., Ltd.
Another company (listed as both a manufacturer and a trader on alibaba) that sells Ni-Fe batteries in China is SeaWill. You *can* buy direct from them.
Cost
- $4k for a 4.3kWhr usable capacity battery - see [9].
- Note that if this is authentically 4.3kW and it lasts 30years - then this is 1/2 the cost of a 24kWhr $4k forklift battery. A forklift battery will last 5 years [10] - so this is 1/6 the length of the NiFe lifetime - or equivalent 4kW over the same time period. However, the beyondoilsolar.com link above says that electrolyte replacement at 30 years gets you another 30 years. If that is true, then we have 60 years life - and 1/2 the cost of lead acid batteries.
- Disadvantage is slow discharge at C/2 rate max. For a 100A bat at 48V, that is 2400W. Plenty.
- Summary - the up-front cost is steep - but lifetime considerations make this a very attractive offer.
- Once open sourced, cost should go down 2x-5x still.
- If we use Lead Acid for long life - 10% DoD - 2.4kW - for 20 year life - that is 3x more expensive than Nickel-Iron batteries over a 30 year life, and 6x more expensive over a 60 year life.
Links
- Good technical description on construction, including patents - Edison Battery
- Energy density is 13 Whr/lb. Compare to Li-Ion at 10x this.
- Paper on reconditioning 85 year old batteries - [11]
- Critique of Nickel Iron batteries - [12] - says that deep discharge destroys them.
- Considerations for NiFe as starting batteries - Nickel-Iron SLI Battery
- Nickel-Iron Battery/Prototype
- Emails and communications - Battery Collaboration
- See also Category:Nickel-Iron Battery Prototypes
- Nickel-Iron Battery/Chemistry
- Detailed construction of the battery is described in the book at Nickel-Iron_Battery/Manufacturing_Instructions - this is the best study of industry standards and taking off point for development. P. 14 in the PDF shows construction details of the battery.